Physics:Stokes line
This article provides insufficient context for those unfamiliar with the subject. (January 2013) (Learn how and when to remove this template message) |
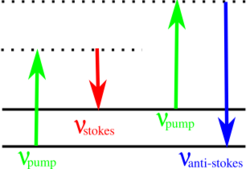
A Stokes line is the radiation of particular wavelengths present in the line spectra associated with fluorescence and the Raman scattering. Stokes lines are of longer wavelength than that of the exciting radiation responsible for the fluorescence or Raman effect.[1][2] Stokes lines are named after Sir George Gabriel Stokes.
The energy of the scattered radiation is less than the incident radiation for the Stokes line, and the energy of the scattered radiation is more than the incident radiation for the anti-Stokes line. The energy increase or decrease from the excitation is related to the vibrational energy spacing in the ground electronic state of the molecule, and therefore the wavenumber of the Stokes and anti-Stokes lines are a direct measure of the vibrational energies of the molecule. The probability of an anti-Stokes transition is much less than that of a Stokes transition, since at room temperature the majority of molecules present are in the lowest vibrational level.
See also
References
- ↑ Banwell C.N. and McCash E.M. Fundamentals of Molecular Spectroscopy (4th ed., McGraw-Hill 1994) p.101 and p.113 ISBN 0-07-707976-0
- ↑ Atkins P. and de Paula J. Physical Chemistry (8th ed., W.H. Freeman 2006) p.431 ISBN 0-7167-8759-8
