| Display title | Chemistry:Barium sulfide |
| Default sort key | Barium sulfide |
| Page length (in bytes) | 8,360 |
| Namespace ID | 3022 |
| Namespace | Chemistry |
| Page ID | 495002 |
| Page content language | en - English |
| Page content model | wikitext |
| Indexing by robots | Allowed |
| Number of redirects to this page | 0 |
| Counted as a content page | Yes |
| Page image | 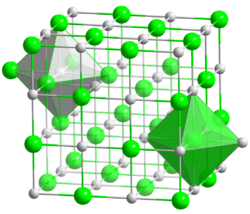 |
| HandWiki item ID | None |
| Edit | Allow all users (infinite) |
| Move | Allow all users (infinite) |
| Page creator | imported>Steve Marsio |
| Date of page creation | 03:02, 9 March 2024 |
| Latest editor | imported>Steve Marsio |
| Date of latest edit | 03:02, 9 March 2024 |
| Total number of edits | 1 |
| Recent number of edits (within past 90 days) | 0 |
| Recent number of distinct authors | 0 |
Description | Content |
Article description: (description)
This attribute controls the content of the description and og:description elements. | Barium sulfide is the inorganic compound with the formula BaS. BaS is the barium compound produced on the largest scale. It is an important precursor to other barium compounds including BaCO3 and the pigment lithopone, ZnS/BaSO4. Like other chalcogenides of the alkaline earth metals, BaS is a short... |

