Chemistry:Tibric acid: Difference between revisions
From HandWiki
John Stpola (talk | contribs) (add) |
(No difference)
|
Latest revision as of 01:07, 6 February 2024
| |
| Names | |
|---|---|
| Preferred IUPAC name
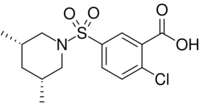
2-Chloro-5-[(3R,5S)-3,5-dimethylpiperidine-1-sulfonyl]benzoic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C14H18ClNO4S | |
| Molar mass | 331.81 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Tibric acid is a sulfamylbenzoic acid that acts as a hypolipidemic agent.[1] Although it was found to be more powerful than clofibrate in lowering lipid levels, it was found to cause liver cancer in mice and rats, and so was not introduced as a human drug.[2] In rats it causes an increase in peroxisomes, and liver enlargement, and then liver cancer. However the peroxisome changes do not occur in humans, and it is not likely to cause liver cancer in humans.[3][4]
Synthesis
Tibric acid can be made in a multi-step process. Firstly 2-chlorobenzoic acid is reacted with chlorosulfonic acid to add a chlorosulfonate group in the 5- position. This reacts with 3,5-dimethylpiperidine to yield tibric acid.[5]
References
- ↑ Bencze, W. L.; Kritchevsky, D.; Dempsey, M. E.; Eisenberg, S.; Felts, J. M.; Frantz, I. D.; Hess, R.; Levy, R. I. et al. (2012). "Hypolipidemic Agents" (in en). Progress in Drug Research / Fortschritte der Arzneimittelforschung / Progrès des recherches pharmaceutiques. 13. Springer Science & Business Media. 217–292. doi:10.1007/978-3-0348-7068-9_5. ISBN 9783642661907. https://books.google.com/books?id=a1bmCAAAQBAJ&pg=PA390.
- ↑ Lalloyer, F.; Staels, B. (14 April 2010). "Fibrates, Glitazones, and Peroxisome Proliferator-Activated Receptors". Arteriosclerosis, Thrombosis, and Vascular Biology 30 (5): 894–899. doi:10.1161/ATVBAHA.108.179689. PMID 20393155.
- ↑ Cohen, A.J.; Grasso, P. (January 1981). "Review of the hepatic response to hypolipidaemic drugs in rodents and assessment of its toxicological significance to man". Food and Cosmetics Toxicology 19 (5): 585–605. doi:10.1016/0015-6264(81)90509-5. PMID 7030887.
- ↑ Lai, David Y. (26 December 2004). "Rodent Carcinogenicity of Peroxisome Proliferators and Issues on Human Relevance". Journal of Environmental Science and Health, Part C 22 (1): 37–55. doi:10.1081/GNC-120038005. PMID 15845221. Bibcode: 2004JESHC..22...37L.
- ↑ Lednicer, Daniel; Mitscher, Lester A.; Georg, Gunda I. (1977) (in en). The Organic Chemistry of Drug Synthesis. John Wiley & Sons. p. 87. ISBN 9780471043928. https://books.google.com/books?id=r-eqWrMoO18C&pg=PA87.
 |

