Chemistry:Vilsmeier–Haack reaction: Difference between revisions
Steve Marsio (talk | contribs) (url) |
(fixing) |
||
| Line 11: | Line 11: | ||
The '''Vilsmeier–Haack reaction''' (also called the '''Vilsmeier reaction''') is the [[Chemistry:Chemical reaction|chemical reaction]] of a substituted [[Chemistry:Formamide|formamide]] ('''1''') with [[Chemistry:Phosphorus oxychloride|phosphorus oxychloride]] and an electron-rich arene ('''3''') to produce an [[Chemistry:Aryl|aryl]] [[Chemistry:Aldehyde|aldehyde]] or [[Chemistry:Ketone|ketone]] ('''5'''): | The '''Vilsmeier–Haack reaction''' (also called the '''Vilsmeier reaction''') is the [[Chemistry:Chemical reaction|chemical reaction]] of a substituted [[Chemistry:Formamide|formamide]] ('''1''') with [[Chemistry:Phosphorus oxychloride|phosphorus oxychloride]] and an electron-rich arene ('''3''') to produce an [[Chemistry:Aryl|aryl]] [[Chemistry:Aldehyde|aldehyde]] or [[Chemistry:Ketone|ketone]] ('''5'''): | ||
:{{awrap|RC({{=}}O)NR{{prime}}R{{pprime}} +}} HArZ + POCl<sub>3</sub> + H<sub>2</sub>O → RC(=O)ArZ + {{awrap|NR{{prime}}R{{pprime}}H +}} HCl + H<sub>3</sub>PO<sub>4</sub> | :{{awrap|RC({{=}}O)NR{{prime}}R{{pprime}} +}} HArZ + POCl<sub>3</sub> + H<sub>2</sub>O → RC(=O)ArZ + {{awrap|NR{{prime}}R{{pprime}}H +}} HCl + H<sub>3</sub>PO<sub>4</sub> | ||
The reaction is named after Anton Vilsmeier and Albrecht Haack.<ref>{{cite journal|last1=Vilsmeier|first1=Anton|last2=Haack|first2=Albrecht|title=Über die Einwirkung von Halogenphosphor auf Alkyl-formanilide. Eine neue Methode zur Darstellung sekundärer und tertiärer ''p''-Alkylamino-benzaldehyde|trans-title=On the reaction of phosphorus halides with alkyl formanilides. A new method for the preparation of secondary and tertiary ''p''-alkylaminobenzaldehydes|journal=Berichte der Deutschen Chemischen Gesellschaft zu Berlin|volume=60|date=1927|pages=119–122|doi=10.1002/cber.19270600118|language=de}}</ref><ref>{{cite journal|last1=Meth-Cohn|first1=O.|last2=Stanforth|first2=S. P.|title=The Vilsmeier–Haack Reaction (Review)|journal=Compr. Org. Synth.|date=1991|volume=2|pages=777–794|doi=10.1016/B978-0-08-052349-1.00049-4}}</ref><ref>{{orgSynth|prep=cv4p0331|title=Formylation of dimethylaniline|last1=Campaigne|first1=E.|last2=Archer|first2=W. L.|collvol=4|collvolpages=331|volume=33|page=27|date=1953|doi=10.15227/orgsyn.033.0027}}</ref> | The reaction is named after Anton Vilsmeier and {{interlanguage link|Albrecht Haack|de}}.<ref>{{cite journal|last1=Vilsmeier|first1=Anton|last2=Haack|first2=Albrecht|title=Über die Einwirkung von Halogenphosphor auf Alkyl-formanilide. Eine neue Methode zur Darstellung sekundärer und tertiärer ''p''-Alkylamino-benzaldehyde|trans-title=On the reaction of phosphorus halides with alkyl formanilides. A new method for the preparation of secondary and tertiary ''p''-alkylaminobenzaldehydes|journal=Berichte der Deutschen Chemischen Gesellschaft zu Berlin|volume=60|date=1927|pages=119–122|doi=10.1002/cber.19270600118|language=de}}</ref><ref>{{cite journal|last1=Meth-Cohn|first1=O.|last2=Stanforth|first2=S. P.|title=The Vilsmeier–Haack Reaction (Review)|journal=Compr. Org. Synth.|date=1991|volume=2|pages=777–794|doi=10.1016/B978-0-08-052349-1.00049-4}}</ref><ref>{{orgSynth|prep=cv4p0331|title=Formylation of dimethylaniline|last1=Campaigne|first1=E.|last2=Archer|first2=W. L.|collvol=4|collvolpages=331|volume=33|page=27|date=1953|doi=10.15227/orgsyn.033.0027}}</ref> | ||
For example, [[Chemistry:Benzanilide|benzanilide]] and [[Chemistry:Dimethylaniline|dimethylaniline]] react with [[Chemistry:Phosphorus oxychloride|phosphorus oxychloride]] to produce an unsymmetrical diaryl ketone.<ref>{{orgSynth|title=Vilsmeyer–Haack reaction of benzanilide and dimethylaniline|last1=Hurd|first1=C. D.|last2=Webb|first2=C. N.|volume=7|page=24|year=1927|doi=10.15227/orgsyn.007.0024}}</ref> Similarly, [[Chemistry:Anthracene|anthracene]] is formylated at the 9-position.<ref>{{orgSynth|title=Formylation of anthracene|last1=Fieser|first1=F. L.|last2=Hartwell|first2=J. L.|last3=Jones|first3=J. E.|last4=Wood|first4=J. H.|last5=Bost|first5=R. W.|volume=20|page=11|year=1940|doi=10.15227/orgsyn.020.0011}}</ref> The reaction of anthracene with ''N''-methylformanilide, also using phosphorus oxychloride, gives 9-anthracenecarboxaldehyde: | For example, [[Chemistry:Benzanilide|benzanilide]] and [[Chemistry:Dimethylaniline|dimethylaniline]] react with [[Chemistry:Phosphorus oxychloride|phosphorus oxychloride]] to produce an unsymmetrical diaryl ketone.<ref>{{orgSynth|title=Vilsmeyer–Haack reaction of benzanilide and dimethylaniline|last1=Hurd|first1=C. D.|last2=Webb|first2=C. N.|volume=7|page=24|year=1927|doi=10.15227/orgsyn.007.0024}}</ref> Similarly, [[Chemistry:Anthracene|anthracene]] is formylated at the 9-position.<ref>{{orgSynth|title=Formylation of anthracene|last1=Fieser|first1=F. L.|last2=Hartwell|first2=J. L.|last3=Jones|first3=J. E.|last4=Wood|first4=J. H.|last5=Bost|first5=R. W.|volume=20|page=11|year=1940|doi=10.15227/orgsyn.020.0011}}</ref> The reaction of anthracene with ''N''-methylformanilide, also using phosphorus oxychloride, gives 9-anthracenecarboxaldehyde: | ||
:[[Image:Vilsmeier reaction example2.svg|thumb|650px|none|''N''-Methylformanilide and anthracene and phosphorus oxychloride]] | :[[Image:Vilsmeier reaction example2.svg|thumb|650px|none|''N''-Methylformanilide and anthracene and phosphorus oxychloride]] | ||
In general, the electron-rich arene ('''3''') must be much more active than benzene for the reaction to proceed; phenols or anilines are good substrates.<ref>{{cite book|page=664|title=March's Organic Chemistry|edition=8th|first1=Michael B.|last1=Smith|publisher=Wiley|year=2020}}</ref> | |||
== Reaction mechanism == | == Reaction mechanism == | ||
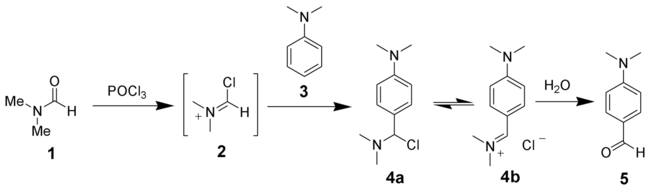
The reaction of a substituted amide with phosphorus oxychloride gives a substituted chloroiminium ion ('''2'''), also called the [[Chemistry:Vilsmeier reagent|Vilsmeier reagent]]. The initial product is an iminium ion ('''4b'''), which is hydrolyzed to the corresponding [[Chemistry:Ketone|ketone]] or [[Chemistry:Aldehyde|aldehyde]] during workup.<ref>{{cite journal|title = The Vilsmeier Reaction of Non-Aromatic Compounds|first1 = G.|last1 = Jones|first2 = S. P.|last2 = Stanforth|journal = Org. React.|year = 2000|volume = 56|issue = 2|pages = 355–686|doi = 10.1002/0471264180.or056.02}}</ref> | The reaction of a substituted amide with phosphorus oxychloride gives a substituted chloroiminium ion ('''2'''), also called the [[Chemistry:Vilsmeier reagent|Vilsmeier reagent]]. The initial product is an iminium ion ('''4b'''), which is hydrolyzed to the corresponding [[Chemistry:Ketone|ketone]] or [[Chemistry:Aldehyde|aldehyde]] during workup.<ref>{{cite journal|title = The Vilsmeier Reaction of Non-Aromatic Compounds|first1 = G.|last1 = Jones|first2 = S. P.|last2 = Stanforth|journal = Org. React.|year = 2000|volume = 56|issue = 2|pages = 355–686|doi = 10.1002/0471264180.or056.02}}</ref> | ||
:[[Image:Vilsmeier Haack Reaction Scheme.png|thumb|none|650px|The Vilsmeier–Haack reaction]] | :[[Image:Vilsmeier Haack Reaction Scheme.png|thumb|none|650px|The Vilsmeier–Haack reaction]] | ||
| Line 28: | Line 29: | ||
*{{cite journal|title=Practical and Efficient Synthesis of Tris(4-formylphenyl)amine, a Key Building Block in Materials Chemistry|date=2005|first1=T.|last1=Mallegol|first2=S.|last2=Gmouh|first3=M.|last3=Aït Amer Meziane|first4=M.|last4=Blanchard-Desce|first5=O.|last5=Mongin|journal=Synthesis|volume=2005|issue=11|pages=1771–1774|doi=10.1055/s-2005-865336}} | *{{cite journal|title=Practical and Efficient Synthesis of Tris(4-formylphenyl)amine, a Key Building Block in Materials Chemistry|date=2005|first1=T.|last1=Mallegol|first2=S.|last2=Gmouh|first3=M.|last3=Aït Amer Meziane|first4=M.|last4=Blanchard-Desce|first5=O.|last5=Mongin|journal=Synthesis|volume=2005|issue=11|pages=1771–1774|doi=10.1055/s-2005-865336}} | ||
*{{cite journal|title=Addition of Tethered Nonaromatic Carbon Nucleophiles to Chemoselectively Activated Amides|last1=Bélanger|first1=G.|last2=Larouche-Gauthier|first2=R.|last3=Ménard|first3=F.|last4=Nantel|first4=M.|last5=Barabé|first5=F.|journal=Org. Lett.|date=2005|volume=7|issue=20|pages=4431–4|doi=10.1021/ol0516519|pmid=16178551|hdl=11143/17289|hdl-access=free}} | *{{cite journal|title=Addition of Tethered Nonaromatic Carbon Nucleophiles to Chemoselectively Activated Amides|last1=Bélanger|first1=G.|last2=Larouche-Gauthier|first2=R.|last3=Ménard|first3=F.|last4=Nantel|first4=M.|last5=Barabé|first5=F.|journal=Org. Lett.|date=2005|volume=7|issue=20|pages=4431–4|doi=10.1021/ol0516519|pmid=16178551|hdl=11143/17289|hdl-access=free}} | ||
* A widely-recommended procedure: {{doi|10.1055/sos-SD-213-00191}} | |||
== References == | == References == | ||
Latest revision as of 21:28, 25 June 2025
| Vilsmeier–Haack reaction | |
|---|---|
| Named after | Anton Vilsmeier Albrecht Haack |
| Reaction type | Substitution reaction |
| Identifiers | |
| Organic Chemistry Portal | vilsmeier-reaction |
| RSC ontology ID | RXNO:0000055 |
The Vilsmeier–Haack reaction (also called the Vilsmeier reaction) is the chemical reaction of a substituted formamide (1) with phosphorus oxychloride and an electron-rich arene (3) to produce an aryl aldehyde or ketone (5):
- RC(=O)NR′R″ + HArZ + POCl3 + H2O → RC(=O)ArZ + NR′R″H + HCl + H3PO4
The reaction is named after Anton Vilsmeier and Albrecht Haack.[1][2][3]
For example, benzanilide and dimethylaniline react with phosphorus oxychloride to produce an unsymmetrical diaryl ketone.[4] Similarly, anthracene is formylated at the 9-position.[5] The reaction of anthracene with N-methylformanilide, also using phosphorus oxychloride, gives 9-anthracenecarboxaldehyde:
In general, the electron-rich arene (3) must be much more active than benzene for the reaction to proceed; phenols or anilines are good substrates.[6]
Reaction mechanism
The reaction of a substituted amide with phosphorus oxychloride gives a substituted chloroiminium ion (2), also called the Vilsmeier reagent. The initial product is an iminium ion (4b), which is hydrolyzed to the corresponding ketone or aldehyde during workup.[7]
See also
- Formylation reaction
Further reading
- Mallegol, T.; Gmouh, S.; Aït Amer Meziane, M.; Blanchard-Desce, M.; Mongin, O. (2005). "Practical and Efficient Synthesis of Tris(4-formylphenyl)amine, a Key Building Block in Materials Chemistry". Synthesis 2005 (11): 1771–1774. doi:10.1055/s-2005-865336.
- Bélanger, G.; Larouche-Gauthier, R.; Ménard, F.; Nantel, M.; Barabé, F. (2005). "Addition of Tethered Nonaromatic Carbon Nucleophiles to Chemoselectively Activated Amides". Org. Lett. 7 (20): 4431–4. doi:10.1021/ol0516519. PMID 16178551.
- A widely-recommended procedure: doi:10.1055/sos-SD-213-00191
References
- ↑ Vilsmeier, Anton; Haack, Albrecht (1927). "Über die Einwirkung von Halogenphosphor auf Alkyl-formanilide. Eine neue Methode zur Darstellung sekundärer und tertiärer p-Alkylamino-benzaldehyde" (in de). Berichte der Deutschen Chemischen Gesellschaft zu Berlin 60: 119–122. doi:10.1002/cber.19270600118.
- ↑ Meth-Cohn, O.; Stanforth, S. P. (1991). "The Vilsmeier–Haack Reaction (Review)". Compr. Org. Synth. 2: 777–794. doi:10.1016/B978-0-08-052349-1.00049-4.
- ↑ Campaigne, E.; Archer, W. L.. "Formylation of dimethylaniline". Organic Syntheses 33: 27. doi:10.15227/orgsyn.033.0027. http://www.orgsyn.org/demo.aspx?prep=cv4p0331.; Collective Volume, 4, pp. 331
- ↑ Hurd, C. D.; Webb, C. N. (1927). "Vilsmeyer–Haack reaction of benzanilide and dimethylaniline". Organic Syntheses 7: 24. doi:10.15227/orgsyn.007.0024.
- ↑ Fieser, F. L.; Hartwell, J. L.; Jones, J. E.; Wood, J. H.; Bost, R. W. (1940). "Formylation of anthracene". Organic Syntheses 20: 11. doi:10.15227/orgsyn.020.0011.
- ↑ Smith, Michael B. (2020). March's Organic Chemistry (8th ed.). Wiley. p. 664.
- ↑ Jones, G.; Stanforth, S. P. (2000). "The Vilsmeier Reaction of Non-Aromatic Compounds". Org. React. 56 (2): 355–686. doi:10.1002/0471264180.or056.02.
 |