Chemistry:Plumbagin
From HandWiki
Revision as of 07:50, 21 September 2021 by imported>MainAI (over-write)
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
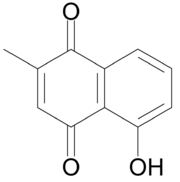
5-Hydroxy-2-methylnaphthalene-1,4-dione | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C11H8O3 | |
| Molar mass | 188.17942 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Plumbagin or 5-hydroxy-2-methyl-1,4-naphthoquinone is an organic compound with the chemical formula C11H8O3. It is regarded as a toxin[1] and it is genotoxic[2] and mutagenic.[3]
Plumbagin is a yellow dye,[1] formally derived from naphthoquinone.
It is named after the plant genus Plumbago, from which it was originally isolated.[4] It is also commonly found in the carnivorous plant genera Drosera and Nepenthes.[5][6] It is also a component of the black walnut drupe.
See also
References
- ↑ 1.0 1.1 Black Walnut. Drugs.com.
- ↑ Jemal Demma; Karl Hallberg; Björn Hellman (2009). "Genotoxicity of plumbagin and its effects on catechol and NQNO-induced DNA damage in mouse lymphoma cells". Toxicology in Vitro 23 (2): 266–271. doi:10.1016/j.tiv.2008.12.007. PMID 19124069.
- ↑ S B Farr; D O Natvig; T Kogoma (1985). "Toxicity and mutagenicity of plumbagin and the induction of a possible new DNA repair pathway in Escherichia coli". J Bacteriol 164 (3): 1309–1316. doi:10.1128/JB.164.3.1309-1316.1985. PMID 2933393.
- ↑ van der Vijver; L. M. (1972). "Distribution of Plumbagin in the Plumbaginaceae". Phytochemistry 11 (11): 3247–3248. doi:10.1016/S0031-9422(00)86380-3.
- ↑ Wang, W.; Luo, X.; Li, H. (2010). "Terahertz and Infrared Spectra of Plumbagin, Juglone, and Menadione". Carnivorous Plant Newsletter 39 (3): 82–88.
- ↑ Rischer, H.; Hamm, A.; Bringmann, G. (2002). "Nepenthes insignis Uses a C2-Portion of the Carbon Skeleton of L-Alanine Acquired via its Carnivorous Organs, to Build up the Allelochemical Plumbagin". Phytochemistry 59 (6): 603–609. doi:10.1016/S0031-9422(02)00003-1. PMID 11867092.
 |

