Chemistry:Xerocomorubin
| |
| Names | |
|---|---|
| IUPAC name
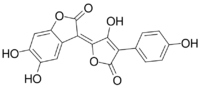
5,6-Dihydroxy-3-[3-hydroxy-4-(4-hydroxyphenyl)-5-oxo-2(5H)-furanylidene]-2(3H)-benzofuranone
| |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| C18H10O8 | |
| Molar mass | 354.270 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Xerocomorubin is a pigment from the fungus order Boletales. It is the oxidized form of isoxerocomic acid.[1] Air oxidation is responsible its formation, and it oxidizes faster to a similar pulvinic acid type pigment oxidized variant, variegatorubin.[1][2] The long wavelength has an absorption at 497 nm, 106 nm higher than its precursor isoxerocomic acid. Synthesis experiments have shown tetra-acetylation by acetic anhydride and sulfuric acid. Although xerocomorubin and variegatorubin give off the same deep red color and could simultaneously occur in a mushroom, extracts from the deep red colored mushroom Boletus rubellus Krombh. identified only variegatorubin by thin layer chromatography (TLC), leading to the question the natural abundance of xerocomorubin.
References
- ↑ 1.0 1.1 Gill, M., and Steglich, W. (1987). "Pigments of fungi (Macromycetes)". Prog Chem Org Nat Prod. Fortschritte der Chemie organischer Naturstoffe / Progress in the Chemistry of Organic Natural Products 51: 1–317. doi:10.1007/978-3-7091-6971-1_1. ISBN 978-3-7091-7456-2. PMID 3315906.
- ↑ Edwards and Gill (1973). Constituents of the Higher Fungi. Part X1l.l Identification of lnvolutin as (-)-cis-5-(3,4-Dihydroxyphenyl)-3,4-dihydroxy-2-(4-hydroxyphenyl)-cyclopent-2-enone and Synthesis of (+)-cis-lnvolutin Trimethyl Ether from Isoxerocomic Acid Derivatives.
 |

