Biology:Hac1 Xbp1 intron
The Hac1 Xbp1 intron is a non-canonical intron, spliced from bZIP-containing genes called HAC1 in Fungi and XBP1 in Metazoa. Splicing is performed independently of the spliceosome by IRE1, a kinase with endoribonuclease activity.[1] Exons are joined by a tRNA ligase. Recognition of the intron splice sites is mediated by a base-paired secondary structure of the mRNA that forms at the exon/intron boundaries. Splicing of the Hac1/Xbp1 intron is a key regulatory step in the unfolded protein response (UPR). The Ire-mediated unconventional splicing was first described for HAC1 in S. cerevisiae.[1]
Consensus structure
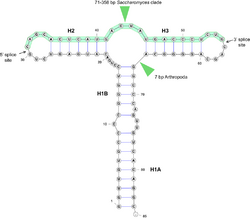
The secondary structure of the HAC1/XBP1 intron is very well conserved, and consists of two hairpins (H2 and H3) around the splice sites, and an extended hairpin (H1) that brings the splice sites together (see figure). The sequence of the intron is well conserved only around the splice sites. Non-canonical splicing motifs CNG'CNG in the loop region of H2 and H3 hairpins are conserved.
The consensus intron is very short (20, 23 or 26 nt). However, 14 yeast species have a long (>100 nt) intron in HAC1.[2] In Saccharomyces cerevisiae the long intron pairs with the 5' UTR and stalls the ribosomes on the mRNA.[3]
Mechanism of splicing
Environmental stress can cause proteins to misfold and aggregate. To protect from these undesirable processes, a cell can activate the unfolded protein response (UPR) pathway. Splicing of HAC1 and XBP1 transcripts is one of the highly regulated ways of activating the UPR in response to presence of unfolded proteins in the endoplasmic reticulum (ER). ER stress activates the endoribonucleolytic activity of IRE1 proteins.[1][4] IRE1 recognizes splice-site motifs in HAC1 or XBP1 transcripts and cleaves them.[1][5] Stem-loop structures around the splice sites and IRE1-specific sequence motifs are both necessary and sufficient for splicing to occur.[1] The joining of exons is performed by tRNA ligase (TRL1 in Saccharomyces cerevisiae).[6]
Intron conservation
IRE-mediated unconventional splicing of the HAC1/XBP1 intron has been confirmed experimentally in the following species:
- S. cerevisiae[1]
- human[4]
- mouse and Caenorhabditis elegans[5]
- Trichoderma reesei and Aspergillus nidulans[7]
- Drosophila melanogaster[8]
- Candida albicans[9]
- Yarrowia lipolytica[10]
- Pichia pastoris.[11]
- Neurospora crassa.[12][13]
Computational methods predict a HAC-like intron with its characteristic RNA structure in 128 out of 156 species studied.[2] In Fungi a HAC-like intron can be found only in Ascomycota (in 52 out of 63 species analysed). All 45 vertebrate genomes analysed, 19 of Arthropoda, 7 of Nematoda, 2 of Annelida and 2 of Mollusca contain a characteristic HAC1-like structure in an open reading frame.[2]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response". Cell 90 (6): 1031–9. 1997. doi:10.1016/S0092-8674(00)80369-4. PMID 9323131.
- ↑ 2.0 2.1 2.2 "Conserved RNA structures in the non-canonical Hac1/Xbp1 intron". RNA Biol 8 (4): 552–556. 2011. doi:10.4161/rna.8.4.15396. PMID 21593604.
- ↑ "Block of HAC1 mRNA translation by long-range base pairing is released by cytoplasmic splicing upon induction of the unfolded protein response". Cell 107 (1): 103–14. 2001. doi:10.1016/S0092-8674(01)00505-0. PMID 11595189.
- ↑ 4.0 4.1 "XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor". Cell 107 (7): 881–91. 2001. doi:10.1016/S0092-8674(01)00611-0. PMID 11779464.
- ↑ 5.0 5.1 "IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA". Nature 415 (6867): 92–6. 2002. doi:10.1038/415092a. PMID 11780124.
- ↑ "tRNA ligase is required for regulated mRNA splicing in the unfolded protein response". Cell 87 (3): 405–413. 1996. doi:10.1016/S0092-8674(00)81361-6. PMID 8898194.
- ↑ "Activation mechanisms of the HAC1-mediated unfolded protein response in filamentous fungi". Mol Microbiol 47 (4): 1149–61. 2003. doi:10.1046/j.1365-2958.2003.03363.x. PMID 12581366.
- ↑ "Unfolded protein response in a Drosophila model for retinal degeneration". EMBO J 26 (1): 242–52. 2007. doi:10.1038/sj.emboj.7601477. PMID 17170705.
- ↑ "Impact of the unfolded protein response upon genome-wide expression patterns, and the role of Hac1 in the polarized growth, of Candida albicans". Fungal Genet Biol 45 (9): 1235–47. 2008. doi:10.1016/j.fgb.2008.06.001. PMID 18602013.
- ↑ "Functional characterization of the unconventional splicing of Yarrowia lipolytica HAC1 mRNA induced by unfolded protein response". Yeast 27 (7): 443–52. 2010. doi:10.1002/yea.1762. PMID 20162530.
- ↑ "The HAC1 gene from Pichia pastoris: characterization and effect of its overexpression on the production of secreted, surface displayed and membrane proteins". Microb Cell Fact 9: 49. 2010. doi:10.1186/1475-2859-9-49. PMID 20591165.
- ↑ "The bZIP Transcription Factor HAC-1 Is Involved in the Unfolded Protein Response and Is Necessary for Growth on Cellulose in Neurospora crassa". PLOS ONE 10 (7): e013141. 2015. doi:10.1371/journal.pone.0131415. PMID 26132395.
- ↑ "Genome-wide analysis of the endoplasmic reticulum stress response during lignocellulase production in Neurospora crassa". Biotechnol Biofuels 8 (66). 2015. doi:10.1186/s13068-015-0248-5. PMID 25883682.