Biology:HNCOCA experiment
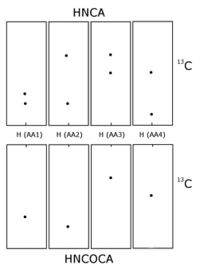
HNCOCA is a 3D triple-resonance NMR experiment commonly used in the field of protein NMR. The name derives from the experiment's magnetization transfer pathway: The magnetization of the amide proton of an amino acid residue is transferred to the amide nitrogen, and then to the alpha carbon of the previous residue in the protein's amino acid sequence. In contrast, the complementary HNCA experiment transfers magnetization to the alpha carbons of both the starting residue and the previous residue in the sequence. The HNCOCA experiment is used, often in tandem with HNCA, to assign alpha carbon resonance signals to specific residues in the protein. This experiment requires a purified sample of protein prepared with 13C and 15N isotopic labelling, at a concentration greater than 0.1 mM, and is thus generally only applied to recombinant proteins.
The spectrum produced by this experiment has 3 dimensions: A proton axis, a 15N axis and a 13C axis. For residue i peaks will appear at {HN(i), N(i), Cα(i-1)} only, while for the complementary HNCA experiment peaks appear at {HN(i), N(i), Cα(i-1)} and {HN(i), N(i), Cα (i)}. Together, these two experiments reveal the alpha carbon chemical shift for each amino acid residue in a protein, and provide information linking adjacent residues in the protein's sequence.
References
Citations
"An efficient 3D NMR technique for correlating the proton and 15N backbone amide resonances with the alpha-carbon of the preceding residue in uniformly 15N/13C enriched proteins". J. Biomol. NMR 1 (1): 99–104. May 1991. doi:10.1007/BF01874573. PMID 1668719.
General references
John Cavanagh; Wayne J. Fairbrother; Arthur G. Palmer III; Nicholas J. Skelton (1995). Protein NMR Spectroscopy : Principles and Practice. Academic Press.
 |