Chemistry:Selenite (ion)
From HandWiki
Short description: Anion composed of selenium and oxygen
Space-filling model of selenite
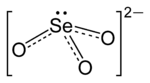
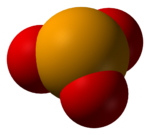
The selenite anion is a selenium oxoanion with the chemical formula SeO2−3.
A selenite (compound) is a compound that contains this ion.
In slightly acid conditions, the hydrogenselenite ion, HSeO−3, is formed; in more acidic conditions selenous acid, H2SeO3, exists.
Most selenite salts can be formed by heating the relevant metal oxide with selenium dioxide, e.g.:
- Na2O + SeO2 → Na2SeO3.
External links