Chemistry:Fischer–Hepp rearrangement
| Fischer-Hepp rearrangement | |
|---|---|
| Named after | Otto Fischer Eduard Hepp |
| Reaction type | Rearrangement reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000095 |
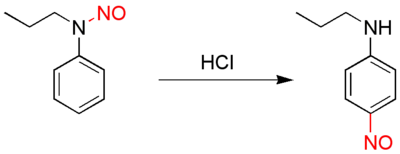
In organic chemistry, the Fischer–Hepp rearrangement is a rearrangement reaction in which an aromatic N-nitroso (–N=O) or nitrosamine (>N–N=O) converts to a carbon nitroso compound:[1][2]
This organic reaction was first described by the German chemist Otto Philipp Fischer (1852–1932) and Eduard Hepp (June 11, 1851 – June 18, 1917) [3] in 1886, and is of importance because para-NO secondary anilines cannot be prepared in a direct reaction.
The rearrangement reaction takes place by reacting the nitrosamine precursor with hydrochloric acid. The chemical yield is generally good under these conditions, but often much poorer if a different acid is used. The exact reaction mechanism is unknown but there is evidence suggesting an intramolecular reaction.
Sources
See also
- Friedel–Crafts alkylation-like reactions:
- Hofmann-Martius rearrangement
- Fries rearrangement
References
 |