Chemistry:N-Sulfinyl imine
N-Sulfinyl imines (N-sulfinylimines, sulfinimines, thiooxime S-oxides) are a class of imines bearing a sulfinyl group attached to nitrogen.[1][2][3][4][5][6][7][8] These imines display useful stereoselectivity reactivity and due to the presence of the chiral electron withdrawing N-sulfinyl group. They allow 1,2-addition of organometallic reagents to imines. The N-sulfinyl group exerts powerful and predictable stereodirecting effects resulting in high levels of asymmetric induction. Racemization of the newly created carbon-nitrogen stereo center is prevented because anions are stabilized at nitrogen (i.e., the sulfinyl group is a versatile amine protection group). The sulfinyl chiral auxiliary is readily removed by simple acid hydrolysis. The addition of organometallic reagents to N-sulfinyl imines is the most reliable and versatile method for the asymmetric synthesis of amine derivatives. These building blocks have been employed in the asymmetric synthesis of numerous biologically active compounds.[1][2][3][4][5][6][7][8]
Synthesis
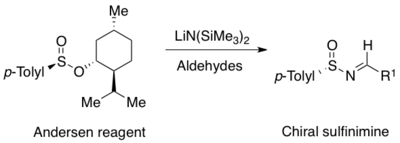
The first N-sulfinyl imines in racemic form were formed by oxidation of p-toluene-sulfenyl imines with m-CPBA.[9] Enantiopure p-toluene-sulfinyl imines arise by the reaction of the commercially available Andersen reagent (menthyl p-toluenesulfinate)[10] with metallo-ketimines but is limited to ketone derived N-sulfinyl imines.[11] A more general method for the preparation of N-sulfinyl imines is the asymmetric oxidation of achiral sulfenyl imines with a chiral oxaziridine.[12] The utility of this method is limited by the availability of the N-sulfonyloxaziridine, which is difficult to prepare.[13] More practical is the one-pot procedure from the Andersen reagent making a variety of p-toluene-sulfinyl imines available from both aromatic and aliphatic aldehydes.[14]
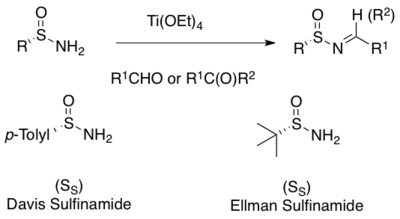
A widely used method for the asymmetric synthesis of N-sulfinyl imines is the condensation of enantiopure primary sulfinamides with aldehyde or ketones.[1][2][3][4][5][6][7] A mild Lewis acid dehydrating reagents such as titanium ethoxide facilitate the condensation.[15][16] Many sulfinamides are commercially available in both (R)- and (S)-forms. The two most commonly used are the Davis p-toluene-sulfinamide and the Ellman tert-butanesulfinamide[8][15][16]
Applications

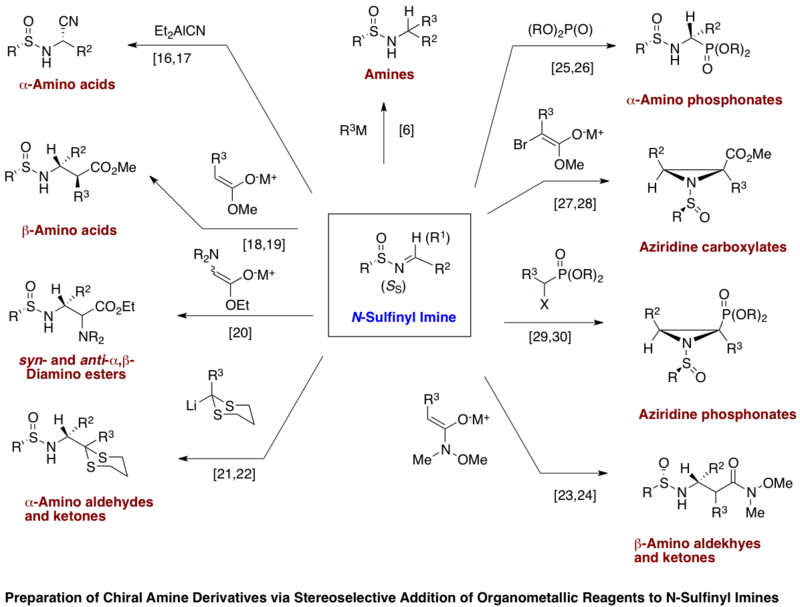
The p-toluene-sulfinyl imines have been used for the highly diastereoselective asymmetric synthesis of α-amino acids,[18][19] β-amino acids,[20][21] syn- and anti-2,3-diamino esters,[22] α-amino aldehydes and ketones,[23][24] β-amino ketones,[25][26] α-amino phosphonates,[27][28] aziridine 2-carboxylates,[29][30] and aziridine 2-phosphonates.[31] Many of these same transformations can be carried out with tert-butylsulfinyl imines.[8] For the asymmetric synthesis of amines, tert-butylsulfinyl imines are required as lithium and Grignard reagents react at the sulfinyl sulfur in p-toluene-sulfinyl imines.[8] Mild acid treatment readily removes the N-sulfinyl group in the sulfinamide products affording the free amine derivatives. An advantage of tert-butylsulfinyl imines is that acid treatment of the corresponding sulfinamides leads to easily removal by-products[8]
References
- ↑ 1.0 1.1 1.2 Davis, Franklin A; Zhou, Ping; Chen, Bang-Chi (1998). "Asymmetric synthesis of amino acids using sulfinimines (thiooxime S-oxides)". Chemical Society Reviews 27: 13. doi:10.1039/A827013Z.
- ↑ 2.0 2.1 2.2 Davis, Franklin A; Zhou, Ping; Chen, Bang-Chi (2004). "Recent advances in asymmetric reactions using sulfinimines (N-sulfinyl imines)". Tetrahedron 60 (37): 8003–8030. doi:10.1016/j.tet.2004.06.071.
- ↑ 3.0 3.1 3.2 Senanayake, Chris H; Krishnamurthy, Dhileepkumar; Luy, Zhi-Hui; Gallou, I (2005). "Enantiopure sulfoxides and sulfinamides: recent developments in their stereoselective synthesis and applications to asymmetric synthesis". Aldrichimica Acta 38 (40): 93. doi:10.1002/chin.200640264.
- ↑ 4.0 4.1 4.2 Morton, Daniel; Stockman, Robert A (2006). "Chiral non-racemic sulfinimines: versatile reagents for asymmetric synthesis". Tetrahedron 62 (38): 8869–8905. doi:10.1016/j.tet.2006.06.107.
- ↑ 5.0 5.1 5.2 Davis, Franklin A (2006). "Adventures in sulfur-nitrogen chemistry". Journal of Organic Chemistry 71 (24): 8993–9003. doi:10.1021/jo061027p. PMID 17109522.
- ↑ 6.0 6.1 6.2 Edupuganti, Ramakrishna; Davis, Franklin A (2012). "Synthesis and Applications of Masked Oxo-Sulfinamides in Asymmetric Synthesis". Organic and Biomolecular Chemistry 10 (26): 5021–31. doi:10.1039/c2ob25345c. PMID 22576951.
- ↑ 7.0 7.1 7.2 Davis, Franklin A; Friedman, Arthur J.; Kluger, Edward W (1974). "Chemistry of the sulfur-Nitrogen Bond. VIII. N-Alkylidenesulfinamides". Journal of the American Chemical Society 96 (15): 5000–5001. doi:10.1021/ja00822a055.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 Ellman, Jonathan; Robak, Maryann T.; Herbage, Melissa A. (2010). "Synthesis and applications of tert-Butanesulfinamide". Chemical Reviews 110 (6): 3600–740. doi:10.1021/cr900382t. PMID 20420386.
- ↑ Hulce, Martin; Mallomo, John P.; Frye, Leah L.; Kogan, Timothy P.; Posner Gary H (1990). Organic Syntheses, Collected Volume 7: 495.
- ↑ Cinquini, Mauro; Cozzi, Franco (1977). "Synthesis of Optically active N-alkylidenesulphinamides". Journal of the Chemical Society, Chemical Communications (14): 502. doi:10.1039/c3977000502b.
- ↑ Davis, Franklin A.; Reddy, Thimma R.; Reddy, Rajaraathnam E. (1992). "Asymmetric Synthesis of Sulfinimines: Applications to the Synthesis of Nonracemic β-Amino acids and α-hydroxy-β-amino acids". Journal of Organic Chemistry 57 (24): 6387–6389. doi:10.1021/jo00050a007.
- ↑ Davis, Franklin A; Reddy, Thimma R.; Han, Wei; Carroll, P. J (1992). "Chemistry of Oxaziridines. 17. N-(Phenylsulfonyl)(3,3-dichlorocamphoryl)oxaziridine: A Highly Efficient Reagent for the Asymmetric Oxidation of Sulfide to Sulfoxides". Journal of the American Chemical Society 114 (4): 1428–1437. doi:10.1021/ja00030a045.
- ↑ Davis, Franklin A.; Reddy, Rajharathnam E.; Szewczyk, Joanna M.; Reddy, G. Venkat; Portonovo, Padma S.; Zhang, Huiming; Fanelli, Dean; Reddy, R. Thimma et al. (1997). "Asymmetric Synthesis and Properties of Sulfinimines (Thiooxime S-Oxides)". Journal of Organic Chemistry 62 (8): 2555–2563. doi:10.1021/jo970077e. PMID 11671597.
- ↑ Liu, Guangcheng; Cogan, Derek. A.; Owens, Timothy D.; Tang, Tony P.; Ellman, Jonathan A. (1999). "Synthesis of Enantiomerically Pure N-tert-Butanesulfinyl Imines (tert-Butanesulfinimines) by the Direct Condensation of tert-Butanesulfinamide with aldehydes and ketones". Journal of Organic Chemistry 64 (4): 1278–1284. doi:10.1021/jo982059i.
- ↑ 15.0 15.1 Davis, Franklin A.; Zhang Yulian; Andemichael, Yemane; Fang, Tianan; Fanelli, Dean L.; Zhang, Huiming (1999). "Improved Synthesis of Enantiopure Sulfinimines (Thiooxime S-Oxides) from p-Toluenesulfinamides and Aldehydes and Ketones". Journal of Organic Chemistry 64 (4): 1403–1406. doi:10.1021/jo9820622.
- ↑ 16.0 16.1 Davis, Franklin A.; Portonovo, Padma S.; Reddy, Rajarathnam E.; Chiu, Yu-hung (1996). "Asymmetric Strecker Synthesis using Enantiopure Sulfinimines and Diethylaluminum Cyanide: The Alcohol Effect". Journal of Organic Chemistry 61 (2): 440–441. doi:10.1021/jo9519928. PMID 11666956.
- ↑ Guo, Tao; Song, Ran; Yuan, Bin-Hua; Chen, Xiao-Yang; Sun, Xing-Wen; Lin, Guo-Qiang (2013). "Highly efficient asymmetric construction of quaternary carbon-containing homoallylic and homopropargylic amines". Chemical Communications 49 (47): 5402–5404. doi:10.1039/C3CC42481B. PMID 23657470.
- ↑ Davis, Franklin A; Srirajan, Vaidyanathan; Titus, Donald D. (1999). "Efficient Asymmetric Synthesis of β-Fluoro α-Amino Acids". Journal of Organic Chemistry 64 (18): 6931–6934. doi:10.1021/jo990947n. PMID 11674714.
- ↑ Davis, Franklin A; Reddy, Rajarathnam E.; Szewczyk, Joanna M. (1995). "Asymmetric Synthesis of (R)-(+)-β-Phenylalanine from (S)-(+)-Benzylidene-p-toluenesulfinamide. Regeneration of the Sulfinimine Precursor". Journal of Organic Chemistry 60 (21): 7037–7039. doi:10.1021/jo00126a070.
- ↑ Davis, Franklin A; Szewczyk, Joanna M.; Reddy, Rajarathnam E (1996). "An Efficient Synthesis of (+)-(S)-Ethyl-β-Amino-3-pyridinepropanoate Using Enantiopure Sulfinimines". Journal of Organic Chemistry 61 (6): 2222–2225. doi:10.1021/jo951917x.
- ↑ Davis, Franklin A; Zhang, Yanfeng; Qiu, Hui (2007). "Asymmetric Synthesis of anti- and syn-2,3-Diamino Esters using Sulfinimines. Water and Concentration Effects". Organic Letters 9 (5): 833–836. doi:10.1021/ol063058c. PMID 17261004.
- ↑ Davis, Franklin A.; Ramachandar, Tokala; Liu Hu (2004). "Asymmetric Synthesis of α-Amino 1,3-Dithioketals from Sulfinimines (N-Sulfinyl Imines). Synthesis of (2S,3R)-(-)-3-Hydroxy-3-methylproline". Organic Letters 6 (19): 3393–5. doi:10.1021/ol0485971. PMID 15355060.
- ↑ Davis, Franklin A; Ramachandar, Tokala; Chai, Jing; Skucas, Eduardas (2006). "Asymmetric Synthesis of α-Amino Aldehydes from Sulfinimine (N-Sulfinyl Imine)-Derived α-Amino 1,3-Dithianes. Formal Synthesis of (-)-2,3-trans-3,4-cis-Dihydroxy Proline". Tetrahedron Letters 47: 2743. doi:10.1016/j.tetlet.2006.02.092.
- ↑ Davis, Franklin A; Nolt, M. Brad; Wu, Yongzhong; Prasad, Kavirayani R.; Li, Danyang; Yang, Bin; Bowen, Kerisha; Lee, Seung H. et al. (2005). "Asymmetric Synthesis of β-Amino Carbonyl Compounds with N-Sulfinyl β-Amino Weinreb Amides". Journal of Organic Chemistry 70 (6): 2184–2190. doi:10.1021/jo0402780. PMID 15760203.
- ↑ Davis, Franklin A; Song Minsoo (2007). "Asymmetric Synthesis of syn-α-Substituted β-Amino Ketones using Sulfinimines and Prochiral Weinreb Amide Enolates". Organic Letters 9 (12): 2413–2416. doi:10.1021/ol0708166. PMID 17497798.
- ↑ Davis, Franklin A; Lee, Seung; Yan, Hongxing; Titus, Donald D. (2001). "Asymmetric Synthesis of Quaternary α-Amino Phosphonates using Sulfinimines". Organic Letters 3 (11): 1757–1760. doi:10.1021/ol015945f. PMID 11405704.
- ↑ Davis, Franklin A.; Lee, Seung; Xu, He (2004). "Asymmetric Synthesis of Cyclic α-Amino Phosphonates using Masked Oxo Sulfinimines (N-Sulfinyl Imines)". Journal of Organic Chemistry 69 (11): 3777. doi:10.1021/jo040127x. PMID 15153008.
- ↑ Davis, Franklin A.; Zhou, Ping; Reddy, G. Venkat (1994). "Asymmetric Synthesis and Reactions of cis-N-(p-Toluenesulfinyl)aziridine-2-carboxylic Acids". Journal of Organic Chemistry 59 (12): 3243–3245. doi:10.1021/jo00091a001.
- ↑ Davis, Franklin A.; Liu, Hu; Zhou, Ping; Fang, Tianan; Reddy, G. Venkat; Zhang, Yulian (1999). "Aza-Darzens Asymmetric Synthesis of N-p-(Toluenesulfinyl)aziridine 2-Carboxylate Esters from Sulfinimines (N-Sulfinyl Imines)". Journal of Organic Chemistry 64 (20): 7559–7567. doi:10.1021/jo990907j.
- ↑ Davis, Franklin A; McCoull, William; Titus, Donald D. (1999). "Asymmetric Synthesis of α-Methylphosphophenylalanine Derivatives using Sulfinimine Derived Chiral Enantiopure Aziridinyl-2-phosphonates". Organic Letters 1 (7): 1053–5. doi:10.1021/ol990855k. PMID 10825956.
- ↑ Davis, Franklin A.; Wu, Yongzhong; Yan, Hongxing; McCoull, William; Prasad, Kavirayani R. (2003). "Asymmetric Synthesis of Aziridine 2-Phosphonates from Enantiopure Sulfinimines (N-Sulfinyl Imines). Synthesis of α-Amino Phosphonates". Journal of Organic Chemistry 68 (6): 2410–2419. doi:10.1021/jo020707z. PMID 12636410.
 |