Chemistry:Phosphorane
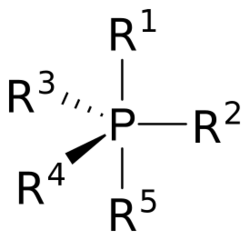
A phosphorane (IUPAC name: λ5-phosphane) is a functional group in organophosphorus chemistry with pentavalent phosphorus. It has the general formula PR5. The parent hydride compound is the hypothetical molecule PH5. The derivative pentaphenylphosphorane (Ph5P) is stable.[1]
Phosphoranes adopt a trigonal bipyramidal molecular geometry with the two apical bonds longer than the three equatorial bonds. Hypervalent bonding is described by inclusion of non-bonding MOs, as also invoked for the closely related molecule phosphorus pentafluoride.[2]
Phosphoranes of the type R3P=CR2 are more common and more important. Phosphoranes are also considered to be one of the resonance structures of ylides, these compounds feature a tetrahedral phosphorus center including a phosphorus–carbon double bond. These compounds are used as reagents in the Wittig reaction, for instance methylenetriphenylphosphorane or Ph3P=CH2.
See also
- Organophosphorus chemistry
- Phosphane
References