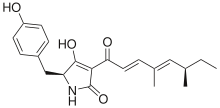
Chemistry:Pretenellin A
| |
| Names | |
|---|---|
| IUPAC name
(1E,2E,4E)-1-[5-[(4-hydroxyphenyl)methyl]-2,4-dioxopyrrolidin-3-ylidene]-4,6-dimethylocta-2,4-dien-1-olate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Related compounds | |
Related compounds
|
Pretenellin B |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pretenellin A is a secondary metabolite in Aspergillus oryzae.[1] Pretenellin A is a substrate for tenellin because it undergoes an oxidative ring expansion to form Pretenellin B followed by N-hydroxylation to form Tenellin, an iron chelator in entomopathegnic fungus.[2]
Biosynthesis
Pretenellin A is biosynthesized using acetate in the initiation module. The acetate molecule is then extended four times by iterative PKS (Figure 1). The specific methylations occur after the first and second elongation module. After the four elongation module, the β-ketopentaketide is brought into close proximity with tyrosine which is attached to a non-ribosomal peptide synthetase (NRPS) (Figure 2). When the iterative PKS interacts with the NRPS system Pretenellin A is released, acting as a well-regulated off-loading mechanism thus releasing Pretenellin A.[1][3]

References
- ↑ 1.0 1.1 Yakasai, Ahmed A.; Davison, Jack; Wasil, Zahida; Halo, Laura M.; Butts, Craig P.; Lazarus, Colin M.; Bailey, Andrew M.; Simpson, Thomas J. et al. (2011-07-20). "Nongenetic Reprogramming of a Fungal Highly Reducing Polyketide Synthase" (in en). Journal of the American Chemical Society 133 (28): 10990–10998. doi:10.1021/ja204200x. ISSN 0002-7863. PMID 21675761. https://pubs.acs.org/doi/10.1021/ja204200x.
- ↑ Jirakkakul, Jiraporn; Cheevadhanarak, Supapon; Punya, Juntira; Chutrakul, Chanikul; Senachak, Jittisak; Buajarern, Taridaporn; Tanticharoen, Morakot; Amnuaykanjanasin, Alongkorn (2015-01-01). "Tenellin acts as an iron chelator to prevent iron-generated reactive oxygen species toxicity in the entomopathogenic fungus Beauveria bassiana" (in en). FEMS Microbiology Letters 362 (2): 1–8. doi:10.1093/femsle/fnu032. ISSN 1574-6968. PMID 25670702. https://academic.oup.com/femsle/article/362/2/1/2467492.
- ↑ Fisch, Katja Maria (2013-09-27). "Biosynthesis of natural products by microbial iterative hybrid PKS–NRPS" (in en). RSC Advances 3 (40): 18228–18247. doi:10.1039/C3RA42661K. ISSN 2046-2069. Bibcode: 2013RSCAd...318228F.
 |


