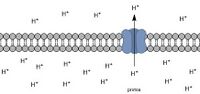
Biology:Intracellular pH
Intracellular pH (pHi) is the measure of the acidity or basicity (i.e., pH) of intracellular fluid. The pHi plays a critical role in membrane transport and other intracellular processes. In an environment with the improper pHi, biological cells may have compromised function.[1][2] Therefore, pHi is closely regulated in order to ensure proper cellular function, controlled cell growth, and normal cellular processes.[3] The mechanisms that regulate pHi are usually considered to be plasma membrane transporters of which two main types exist — those that are dependent and those that are independent of the concentration of bicarbonate (HCO−3). Physiologically normal intracellular pH is most commonly between 7.0 and 7.4, though there is variability between tissues (e.g., mammalian skeletal muscle tends to have a pHi of 6.8–7.1).[4][5] There is also pH variation across different organelles, which can span from around 4.5 to 8.0.[6][7] pHi can be measured in a number of different ways.[3][8]
Homeostasis
Intracellular pH is typically lower than extracellular pH due to lower concentrations of HCO3−.[9] A rise of extracellular (e.g., serum) partial pressure of carbon dioxide (pCO2) above 45 mmHg leads to formation of carbonic acid, which causes a decrease of pHi as it dissociates:[10]
- H2O + CO2 ⇌ H2CO3 ⇌ H+ + HCO3–
Since biological cells contain fluid that can act as a buffer, pHi can be maintained fairly well within a certain range.[11] Cells adjust their pHi accordingly upon an increase in acidity or basicity, usually with the help of CO2 or HCO3– sensors present in the membrane of the cell.[3] These sensors can permit H+ to pass through the cell membrane accordingly, allowing for pHi to be interrelated with extracellular pH in this respect.[12]
Major intracellular buffer systems include those involving proteins or phosphates. Since the proteins have acidic and basic regions, they can serve as both proton donors or acceptors in order to maintain a relatively stable intracellular pH. In the case of a phosphate buffer, substantial quantities of weak acid and conjugate weak base (H2PO4– and HPO42–) can accept or donate protons accordingly in order to conserve intracellular pH:[13][14]
- OH– + H2PO4– ⇌ H2O + HPO42–
- H+ + HPO42– ⇌ H2PO4–
In organelles
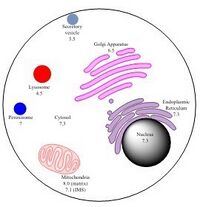
The pH within a particular organelle is tailored for its specific function.
For example, lysosomes have a relatively low pH of 4.5.[6] Additionally, fluorescence microscopy techniques have indicated that phagocytes also have a relatively low internal pH.[15] Since these are both degradative organelles that engulf and break down other substances, they require high internal acidity in order to successfully perform their intended function.[15]
In contrast to the relatively low pH inside lysosomes and phagocytes, the mitochondrial matrix has an internal pH of around 8.0, which is approximately 0.9 pH units higher than that of inside intermembrane space.[6][16] Since oxidative phosphorylation must occur inside the mitochondria, this pH discrepancy is necessary to create a gradient across the membrane. This membrane potential is ultimately what allows for the mitochondria to generate large quantities of ATP.[17]
Measurement
There are several common ways in which intracellular pH (pHi) can be measured including with a microelectrode, dye that is sensitive to pH, or with nuclear magnetic resonance techniques.[18][19] For measuring pH inside of organelles, a technique utilizing pH-sensitive green fluorescent proteins (GFPs) may be used.[20]
Overall, all three methods have their own advantages and disadvantages. Using dyes is perhaps the easiest and fairly precise, while NMR presents the challenge of being relatively less precise.[18] Furthermore, using a microelectrode may be challenging in situations where the cells are too small, or the intactness of the cell membrane should remain undisturbed.[19] GFPs are unique in that they provide a noninvasive way of determining pH inside different organelles, yet this method is not the most quantitatively precise way of determining pH.[21]
Microelectrode
The microelectrode method for measuring pHi consists of placing a very small electrode into the cell’s cytosol by making a very small hole in the plasma membrane of the cell.[19] Since the microelectrode has fluid with a high H+ concentration inside, relative to the outside of the electrode, there is a potential created due to the pH discrepancy between the inside and outside of the electrode.[18][19] From this voltage difference, and a predetermined pH for the fluid inside the electrode, one can determine the intracellular pH (pHi) of the cell of interest.[19]
Fluorescence spectroscopy
Another way to measure Intracellular pH (pHi) is with dyes that are sensitive to pH, and fluoresce differently at various pH values.[15][22] This technique, which makes use of fluorescence spectroscopy, consists of adding this special dye to the cytosol of a cell.[18][19] By exciting the dye in the cell with energy from light, and measuring the wavelength of light released by the photon as it returns to its native energy state, one can determine the type of dye present, and relate that to the intracellular pH of the given cell.[18][19]
Nuclear magnetic resonance
In addition to using pH-sensitive electrodes and dyes to measure pHi, Nuclear Magnetic Resonance (NMR) spectroscopy can also be used to quantify pHi.[19] NMR, typically speaking, reveals information about the inside of a cell by placing the cell in an environment with a potent magnetic field.[18][19] Based on the ratio between the concentrations of protonated, compared to deprotonated, forms of phosphate compounds in a given cell, the internal pH of the cell can be determined.[18] Additionally, NMR may also be used to reveal the presence of intracellular sodium, which can also provide information about the pHi.[23]
Using NMR Spectroscopy, it has been determined that lymphocytes maintain a constant internal pH of 7.17± 0.06, though, like all cells, the intracellular pH changes in the same direction as extracellular pH.[24]
pH-sensitive GFPs
To determine the pH inside organelles, pH-sensitive GFPs are often used as part of a noninvasive and effective technique.[20] By using cDNA as a template along with the appropriate primers, the GFP gene can be expressed in the cytosol, and the proteins produced can target specific regions within the cell, such as the mitochondria, golgi apparatus, cytoplasm, and endoplasmic reticulum.[21] If certain GFP mutants that are highly sensitive to pH in intracellular environments are used in these experiments, the relative amount of resulting fluorescence can reveal the approximate surrounding pH.[21][25]
References
- ↑ Harguindey, S; Stanciu, D; Devesa, J; Alfarouk, K; Cardone, RA; Polo Orozco, JD; Devesa, P; Rauch, C et al. (April 2017). "Cellular acidification as a new approach to cancer treatment and to the understanding and therapeutics of neurodegenerative diseases.". Seminars in Cancer Biology 43: 157–179. doi:10.1016/j.semcancer.2017.02.003. PMID 28193528. http://eprints.nottingham.ac.uk/43674/.
- ↑ "Roles of pH in control of cell proliferation". Acta Physiologica 223 (3): e13068. July 2018. doi:10.1111/apha.13068. PMID 29575508.
- ↑ 3.0 3.1 3.2 "Regulation of intracellular pH". Advances in Physiology Education 28 (1–4): 160–79. December 2004. doi:10.1152/advan.00045.2004. PMID 15545345.
- ↑ Brandis, Kerry. "2.6 Regulation of Intracellular Hydrogen Ion Concentration". Acid-Base Physiology. Anaesthesia Education Website. https://www.anaesthesiamcq.com/AcidBaseBook/ab2_6.php.
- ↑ "Regulation of intracellular pH in eukaryotic cells". The Biochemical Journal 250 (1): 1–8. February 1988. doi:10.1042/bj2500001. PMID 2965576.
- ↑ 6.0 6.1 6.2 6.3 "Exploitation of intracellular pH gradients in the cellular delivery of macromolecules". Journal of Pharmaceutical Sciences 91 (4): 903–13. April 2002. doi:10.1002/jps.10095. PMID 11948528.
- ↑ "pH in nature, humans and skin". The Journal of Dermatology 45 (9): 1044–1052. September 2018. doi:10.1111/1346-8138.14489. PMID 29863755.
- ↑ "Novel probes for pH and dissolved oxygen measurements in cultivations from millilitre to benchtop scale". Applied Microbiology and Biotechnology 100 (9): 3853–63. May 2016. doi:10.1007/s00253-016-7412-0. PMID 26995606.
- ↑ "Roles of pH in control of cell proliferation". Acta Physiol (Oxf) 223 (3): e13068. July 2018. doi:10.1111/apha.13068. PMID 29575508. [verification needed]
- ↑ Flinck M, Kramer SH, Pedersen SF (July 2018). "Roles of pH in control of cell proliferation". Acta Physiol (Oxf). 223 (3): e13068. doi:10.1111/apha.13068. PMID 29575508.
- ↑ "Cytoplasmic pH measurement and homeostasis in bacteria and archaea". Advances in Microbial Physiology 55: 1–79, 317. 2009. doi:10.1016/S0065-2911(09)05501-5. ISBN 9780123747907. PMID 19573695.
- ↑ "Red blood cell pH, the Bohr effect, and other oxygenation-linked phenomena in blood O2 and CO2 transport". Acta Physiologica Scandinavica 182 (3): 215–27. November 2004. doi:10.1111/j.1365-201X.2004.01361.x. PMID 15491402.
- ↑ Bookallil, Michael J. "Acid-Base: pH of the Blood - 3 - Control mechanisms". Lectures and Study Notes Listing. The University of Sydney Nuffield Department of Anaesthetics. http://www.anaesthesia.med.usyd.edu.au/resources/lectures/acidbase_mjb/control.html#co2control.
- ↑ "Intracellular buffering". Respiration Physiology 33 (1): 51–8. April 1978. doi:10.1016/0034-5687(78)90083-X. PMID 27854.
- ↑ 15.0 15.1 15.2 "Measuring Phagosome pH by Ratiometric Fluorescence Microscopy". Journal of Visualized Experiments (106): e53402. December 2015. doi:10.3791/53402. PMID 26710109.
- ↑ "pH difference across the outer mitochondrial membrane measured with a green fluorescent protein mutant". Biochemical and Biophysical Research Communications 326 (4): 799–804. January 2005. doi:10.1016/j.bbrc.2004.11.105. PMID 15607740.
- ↑ "The Mitochondrion". Molecular Biology of the Cell (4th ed.). New York: Garland Science. 2002. https://www.ncbi.nlm.nih.gov/books/NBK26894/.
- ↑ 18.0 18.1 18.2 18.3 18.4 18.5 18.6 "Intracellular pH". Physiological Reviews 61 (2): 296–434. April 1981. doi:10.1152/physrev.1981.61.2.296. PMID 7012859.
- ↑ 19.0 19.1 19.2 19.3 19.4 19.5 19.6 19.7 19.8 "Measurement of Intracellular pH". Membrane Transporters in Drug Discovery and Development. Methods in Molecular Biology. 637. 2010. pp. 311–31. doi:10.1007/978-1-60761-700-6_17. ISBN 978-1-60761-699-3.
- ↑ 20.0 20.1 "Corrigendum: Identification and Characterisation of a pH-stable GFP". Scientific Reports 8: 46976. May 2018. doi:10.1038/srep46976. PMID 29769631. Bibcode: 2018NatSR...846976R.
- ↑ 21.0 21.1 21.2 "Green fluorescent protein as a noninvasive intracellular pH indicator". Biophysical Journal 74 (3): 1591–9. March 1998. doi:10.1016/S0006-3495(98)77870-1. PMID 9512054. Bibcode: 1998BpJ....74.1591K.
- ↑ "A Critical and Comparative Review of Fluorescent Tools for Live-Cell Imaging". Annual Review of Physiology 79: 93–117. February 2017. doi:10.1146/annurev-physiol-022516-034055. PMID 27860833.
- ↑ "Sodium NMR/MRI for anisotropic systems". NMR in Biomedicine 29 (2): 144–52. February 2016. doi:10.1002/nbm.3331. PMID 26105084.
- ↑ "Regulation of intracellular pH by human peripheral blood lymphocytes as measured by 19F NMR". Proceedings of the National Academy of Sciences of the United States of America 79 (24): 7944–8. December 1982. doi:10.1073/pnas.79.24.7944. PMID 6961462. Bibcode: 1982PNAS...79.7944D.
- ↑ "Chimeric green fluorescent protein as a tool for visualizing subcellular organelles in living cells". Current Biology 5 (6): 635–42. June 1995. doi:10.1016/s0960-9822(95)00128-x. PMID 7552174.
 |