Chemistry:Carbonic acid
| |
| |
| Names | |
|---|---|
| IUPAC name
Carbonic acid[1]
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| 25554 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| H2CO3 | |
| Appearance | Colorless gas |
| Melting point | −53 °C (−63 °F; 220 K)[3] (sublimes) |
| Boiling point | 127 °C (261 °F; 400 K) (decomposes) |
| Reacts to form carbon dioxide and water | |
| Acidity (pKa) | |
| Conjugate base | Bicarbonate, carbonate |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Structure | |
| monoclinic | |
| p21/c, No. 14 | |
| - | |
a = 5.392 Å, b = 6.661 Å, c = 5.690 Å α = 90°, β = 92.66°, γ = 90°[4] (D2CO3 at 1.85 GPa, 298 K)
| |
Lattice volume (V)
|
204.12 Å3 |
Formula units (Z)
|
4 formula per cell |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
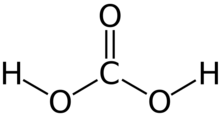
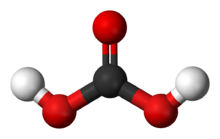
Carbonic acid is a chemical compound with the chemical formula H
2CO
3. The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature.[5][6] The interconversion of carbon dioxide and carbonic acid is related to the breathing cycle of animals and the acidification of natural waters.[4]
In biochemistry and physiology, the name "carbonic acid" is sometimes applied to aqueous solutions of carbon dioxide. These chemical species play an important role in the bicarbonate buffer system, used to maintain acid–base homeostasis.[7]
Terminology in biochemical literature
In chemistry, the term "carbonic acid" strictly refers to the chemical compound with the formula H2CO3. Some biochemistry literature effaces the distinction between carbonic acid and carbon dioxide dissolved in extracellular fluid.
In physiology, carbon dioxide excreted by the lungs may be called volatile acid or respiratory acid.
Anhydrous carbonic acid
At ambient temperatures, pure carbonic acid is a stable gas.[6] There are two main methods to produce anhydrous carbonic acid: reaction of hydrogen chloride and potassium bicarbonate at 100 K in methanol and proton irradiation of pure solid carbon dioxide.[3] Chemically, it behaves as a diprotic Brønsted acid.[8][9]
Carbonic acid monomers exhibit three conformational isomers: cis–cis, cis–trans, and trans–trans.[10]
At low temperature and atmospheric pressure, solid carbonic acid is amorphous and lacks Bragg peaks in X-ray diffraction.[11] But at high pressure, carbonic acid crystallizes, and modern analytical spectroscopy can measure its geometry.
According to neutron diffraction of dideuterated carbonic acid (D2CO3) in a hybrid clamped cell (Russian alloy/copper-beryllium) at 1.85 GPa, the molecules are planar and form dimers joined by pairs of hydrogen bonds. All three C-O bonds are nearly equidistant at 1.34 Å, intermediate between typical C-O and C=O distances (respectively 1.43 and 1.23 Å). The unusual C-O bond lengths are attributed to delocalized π bonding in the molecule's center and extraordinarily strong hydrogen bonds. The same effects also induce a very short O—O separation (2.13 Å), through the 136° O-H-O angle imposed by the doubly hydrogen-bonded 8-membered rings.[4] Longer O—O distances are observed in strong intramolecular hydrogen bonds, e.g. in oxalic acid, where the distances exceed 2.4 Å.[11]
In aqueous solution
In the presence of even a slight amount of water, carbonic acid dehydrates to carbon dioxide and water, which then catalyzes further decomposition.[6] For this reason, carbon dioxide can be considered the carbonic acid anhydride.
The hydration equilibrium constant at 25 °C is [H2CO3]/[CO
2] ≈ 1.7×10−3 in pure water[12] and ≈ 1.2×10−3 in seawater.[13] Hence the majority of carbon dioxide at geophysical or biological air-water interfaces does not convert to carbonic acid, remaining dissolved CO
2 gas. However, the uncatalyzed equilibrium is reached quite slowly: the rate constants are 0.039 s−1 for hydration and 23 s−1 for dehydration.
In biological solutions
In the presence of the enzyme carbonic anhydrase, equilibrium is instead reached rapidly, and the following reaction takes precedence:[14]
When the created carbon dioxide exceeds its solubility, gas evolves and a third equilibrium must also be taken into consideration. The equilibrium constant for this reaction is defined by Henry's law.
The two reactions can be combined for the equilibrium in solution: When Henry's law is used to calculate the denominator care is needed with regard to units since Henry's law constant can be commonly expressed with 8 different dimensionalities.[15]
In water pH control
In wastewater treatment and agriculture irrigation, carbonic acid is used to acidify the water similar to sulfuric acid and sulfurous acid produced by sulfur burners.[16]
Under high CO2 partial pressure
In the beverage industry, sparkling or "fizzy water" is usually referred to as carbonated water. It is made by dissolving carbon dioxide under a small positive pressure in water. Many soft drinks treated the same way effervesce.
Significant amounts of molecular H2CO3 exist in aqueous solutions subjected to pressures of multiple gigapascals (tens of thousands of atmospheres) in planetary interiors.[17][18] Pressures of 0.6–1.6 GPa at 100 K, and 0.75–1.75 GPa at 300 K are attained in the cores of large icy satellites such as Ganymede, Callisto, and Titan, where water and carbon dioxide are present. Pure carbonic acid, being denser than the ice, is expected to have sunk beneath the ice layers and to separate them from the rocky cores of these moons.[19]
Relationship to bicarbonate and carbonate

Carbonic acid is the formal Brønsted–Lowry conjugate acid of the bicarbonate anion, stable in alkaline solution. The protonation constants have been measured to great precision, but depend on overall ionic strength I. The two equilibria most easily measured are as follows: where brackets indicate the concentration of species. At 25 °C, these equilibria empirically satisfy[20]log(β1) decreases with increasing I, as does log(β2). In a solution absent other ions (e.g. I = 0), these curves imply the following stepwise dissociation constants: Direct values for these constants in the literature include pK1 = 6.35 and pK2 - pK1 = 3.49.[21]
To interpret these numbers, note that two chemical species in an acid equilibrium are equiconcentrated when pK = pH. In particular, the extracellular fluid (cytosol) in biological systems exhibits pH ≈ 7.2, so that carbonic acid will be almost 50%-dissociated at equilibrium.
Ocean acidification

The Bjerrum plot shows typical equilibrium concentrations, in solution, in seawater, of carbon dioxide and the various species derived from it, as a function of pH.[8][9] As human industrialization has increased the proportion of carbon dioxide in Earth's atmosphere, the proportion of carbon dioxide dissolved in sea- and freshwater as carbonic acid is also expected to increase. This rise in dissolved acid is also expected to acidify those waters, generating a decrease in pH.[22][23] It has been estimated that the increase in dissolved carbon dioxide has already caused the ocean's average surface pH to decrease by about 0.1 from pre-industrial levels.
Further reading
- Template:WsPSM2
- Welch, M. J.; Lifton, J. F.; Seck, J. A. (1969). "Tracer studies with radioactive oxygen-15. Exchange between carbon dioxide and water". J. Phys. Chem. 73 (335): 3351. doi:10.1021/j100844a033.
- Jolly, W. L. (1991). Modern Inorganic Chemistry (2nd ed.). McGraw-Hill. ISBN 978-0-07-112651-9.
- Moore, M. H.; Khanna, R. (1991). "Infrared and Mass Spectral Studies of Proton Irradiated H2O+CO2 Ice: Evidence for Carbonic Acid Ice: Evidence for Carbonic Acid". Spectrochimica Acta 47A (2): 255–262. doi:10.1016/0584-8539(91)80097-3. Bibcode: 1991AcSpA..47..255M. https://zenodo.org/record/1258549.
- W. Hage, K. R. Liedl; Liedl, E.; Hallbrucker, A; Mayer, E (1998). "Carbonic Acid in the Gas Phase and Its Astrophysical Relevance". Science 279 (5355): 1332–5. doi:10.1126/science.279.5355.1332. PMID 9478889. Bibcode: 1998Sci...279.1332H.
- Hage, W.; Hallbrucker, A.; Mayer, E. (1995). "A Polymorph of Carbonic Acid and Its Possible Astrophysical Relevance". J. Chem. Soc. Faraday Trans. 91 (17): 2823–6. doi:10.1039/ft9959102823. Bibcode: 1995JCSFT..91.2823H.
References
- ↑ "Front Matter". Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. P001–4. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ 2.0 2.1 2.2 Perrin, D. D., ed (1982). Ionisation Constants of Inorganic Acids and Bases in Aqueous Solution. IUPAC Chemical Data (2nd ed.). Oxford: Pergamon (published 1984). "Carbonic Acid, H2CO3" entry. ISBN 0-08-029214-3.
- ↑ 3.0 3.1 W. Hage, K. R. Liedl; Liedl, E.; Hallbrucker, A; Mayer, E (1998). "Carbonic Acid in the Gas Phase and Its Astrophysical Relevance". Science 279 (5355): 1332–5. doi:10.1126/science.279.5355.1332. PMID 9478889. Bibcode: 1998Sci...279.1332H.
- ↑ 4.0 4.1 4.2 Benz, Sebastian; Chen, Da; Möller, Andreas; Hofmann, Michael; Schnieders, David; Dronskowski, Richard (September 2022). "The Crystal Structure of Carbonic Acid" (in en). Inorganics 10 (9): 132. doi:10.3390/inorganics10090132. ISSN 2304-6740.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 310. ISBN 978-0-08-037941-8.
- ↑ 6.0 6.1 6.2 Loerting, Thomas; Tautermann, Christofer; Kroemer, Romano T.; Kohl, Ingrid; Hallbrucker, Andreas; Mayer, Erwin; Liedl, Klaus R.; Loerting, Thomas et al. (2000). "On the Surprising Kinetic Stability of Carbonic Acid (H2CO3)". Angewandte Chemie International Edition 39 (5): 891–4. doi:10.1002/(SICI)1521-3773(20000303)39:5<891::AID-ANIE891>3.0.CO;2-E. PMID 10760883.
- ↑ Acid-Base Physiology 2.1 – Acid-Base Balance by Kerry Brandis.
- ↑ 8.0 8.1 Pangotra, Dhananjai; Csepei, Lénárd-István; Roth, Arne; Ponce de León, Carlos; Sieber, Volker; Vieira, Luciana (2022). "Anodic production of hydrogen peroxide using commercial carbon materials". Applied Catalysis B: Environmental 303. doi:10.1016/j.apcatb.2021.120848. Bibcode: 2022AppCB.30320848P.
- ↑ 9.0 9.1 Andersen, C. B. (2002). "Understanding carbonate equilibria by measuring alkalinity in experimental and natural systems". Journal of Geoscience Education 50 (4): 389–403. doi:10.5408/1089-9995-50.4.389. Bibcode: 2002JGeEd..50..389A.
- ↑ Loerting, Thomas; Bernard, Juergen (2010). "Aqueous Carbonic Acid (H2CO3)". ChemPhysChem 11 (11): 2305–9. doi:10.1002/cphc.201000220. PMID 20397242.
- ↑ 11.0 11.1 Winkel, Katrin; Hage, Wolfgang; Loerting, Thomas; Price, Sarah L.; Mayer, Erwin (2007). "Carbonic Acid: From Polyamorphism to Polymorphism". Journal of the American Chemical Society 129 (45): 13863–71. doi:10.1021/ja073594f. PMID 17944463. Bibcode: 2007JAChS.12913863W.
- ↑ Housecroft, C.E.; Sharpe, A.G. (2005). Inorganic Chemistry (2nd ed.). Prentice-Pearson-Hall. p. 368. ISBN 0-13-039913-2. OCLC 56834315.
- ↑ Soli, A. L.; R. H. Byrne (2002). "CO2 system hydration and dehydration kinetics and the equilibrium CO2/H2CO3 ratio in aqueous NaCl solution". Marine Chemistry 78 (2–3): 65–73. doi:10.1016/S0304-4203(02)00010-5. Bibcode: 2002MarCh..78...65S.
- ↑ "Structure and mechanism of carbonic anhydrase". Pharmacology & Therapeutics 74 (1): 1–20. 1997. doi:10.1016/S0163-7258(96)00198-2. PMID 9336012.
- ↑ Sander, Rolf; Acree, William E.; Visscher, Alex De; Schwartz, Stephen E.; Wallington, Timothy J. (2022-01-01). "Henry's law constants (IUPAC Recommendations 2021)" (in en). Pure and Applied Chemistry 94 (1): 71–85. doi:10.1515/pac-2020-0302. ISSN 1365-3075.
- ↑ Meneses, Adolfo (November 19, 2024). "Irrigation water acidification using captured CO2; An option to traditional acidification systems.". https://www.worldagexpo.com/wp-content/uploads/sites/2/2020/11/Soil-acidification-r.-1.0.pdf.
- ↑ Wang, Hongbo; Zeuschner, Janek; Eremets, Mikhail; Troyan, Ivan; Williams, Jonathon (27 January 2016). "Stable solid and aqueous H2CO3 from CO2 and H2O at high pressure and high temperature". Scientific Reports 6 (1). doi:10.1038/srep19902. PMID 26813580. Bibcode: 2016NatSR...619902W.
- ↑ Stolte, Nore; Pan, Ding (4 July 2019). "Large presence of carbonic acid in CO2-rich aqueous fluids under Earth's mantle conditions". The Journal of Physical Chemistry Letters 10 (17): 5135–41. doi:10.1021/acs.jpclett.9b01919. PMID 31411889.
- ↑ G. Saleh; A. R. Oganov (2016). "Novel Stable Compounds in the C-H-O Ternary System at High Pressure". Scientific Reports 6. doi:10.1038/srep32486. PMID 27580525. Bibcode: 2016NatSR...632486S.
- ↑ IUPAC (2006). "Stability constants" (database).
- ↑ Pines, Dina; Ditkovich, Julia; Mukra, Tzach; Miller, Yifat; Kiefer, Philip M.; Daschakraborty, Snehasis; Hynes, James T.; Pines, Ehud (2016). "How Acidic Is Carbonic Acid?". J Phys Chem B 120 (9): 2440–51. doi:10.1021/acs.jpcb.5b12428. PMID 26862781.
- ↑ Caldeira, K.; Wickett, M. E. (2003). "Anthropogenic carbon and ocean pH". Nature 425 (6956): 365. doi:10.1038/425365a. PMID 14508477. Bibcode: 2001AGUFMOS11C0385C. https://zenodo.org/record/1233227.
- ↑ Sabine, C. L. (2004). "The Oceanic Sink for Anthropogenic CO2". Science 305 (5682): 367–371. doi:10.1126/science.1097403. PMID 15256665. Bibcode: 2004Sci...305..367S. https://www.science.org/doi/abs/10.1126/science.1097403. Retrieved 22 June 2021.
External links
- Carbonic acid/bicarbonate/carbonate equilibrium in water: pH of solutions, buffer capacity, titration, and species distribution vs. pH, computed with a free spreadsheet
- How to calculate concentration of carbonic acid in water
| H2CO3 | He | ||||||||||||||||
| Li2CO3, LiHCO3 |
BeCO3 | B | C | (NH4)2CO3, NH4HCO3 |
O | F | Ne | ||||||||||
| Na2CO3, NaHCO3, Na3H(CO3)2 |
MgCO3, Mg(HCO3)2 |
Al2(CO3)3 | Si | P | S | Cl | Ar | ||||||||||
| K2CO3, KHCO3 |
CaCO3, Ca(HCO3)2 |
Sc | Ti | V | Cr | MnCO3 | FeCO3 | CoCO3 | NiCO3 | CuCO3 | ZnCO3 | Ga | Ge | As | Se | Br | Kr |
| Rb2CO3 | SrCO3 | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag2CO3 | CdCO3 | In | Sn | Sb | Te | I | Xe |
| Cs2CO3, CsHCO3 |
BaCO3 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl2CO3 | PbCO3 | (BiO)2CO3 | Po | At | Rn | |
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La2(CO3)3 | Ce2(CO3)3 | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | Th | Pa | UO2CO3 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||
 |

