Chemistry:Nitropentaamminecobalt(III) chloride

| |
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| H15N6O2Cl2Co | |
| Molar mass | 261.00 g/mol |
| Density | 1.83 g/mL[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
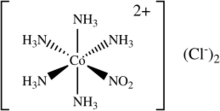
Nitropentaamminecobalt(III) chloride is an inorganic compound with the molecular formula [Co(NH3)5(NO2)]Cl2. It is an orange solid that is soluble in water. Although it has no applications, the compound has been of academic interest as a source of the transition metal nitrite complex [Co(NH3)5(NO2)]2+.
Linkage isomers
The coordination complexes [Co(NH3)5(NO2)]2+ and [Co(NH3)5(ONO)]2+ provided an early example of linkage isomerism. This nitritopentaamminecobalt(III) isomer converts to the more stable nitro form at room temperature.[2] The two isomers can be distinguished by UV-Vis spectroscopy. Absorbance maxima for the nitro isomer occur at 457.5, 325, and 239 nm. The nitrito has maxima at 486, 330, and 220 nm.[3] Their IR spectra also differ. The nitrito isomer absorbs at 1460 and 1065 cm−1. The nitro isomer absorbs at 1430 and 825 cm−1.[4] The O-linkage isomer scrambles rapidly between the two oxygen sites, i.e. Co-O*NO/Co-ONO*.[5]
Preparation and reactions
Nitritopentaamminecobalt(III) chloride is prepared by treating chloropentamminecobalt chloride with sodium nitrite:
- [Co(NH3)5Cl]2+ + NO2− → [Co(NH3)5(ONO)]2+ + Cl−
Heating a solution of the nitrito complex gives the nitro isomer.
Nitropentaamminecobalt(III) chloride has been studied for its ability to repress cell division.[6] This property has been tested to inhibit the growth of tumors and bacteria such as E. coli. However, it has been found that several other compounds are superior inhibitors.
References
- ↑ Grenthe, I; Nordin, E. (1979). "Nitrito-Nitro Linkage Isomerization in the Solid State. 2. A Comparative Study of the Structures of Nitrito- and Nitropentaamminecobalt( 111) Dichloride". Inorganic Chemistry 18 (7): 1869–74. doi:10.1021/ic50197a031.
- ↑ Hohman, W. H. (1974). "A Combined Infrared and Kinetic Study of Linkage Isomers. An Inorganic Experiment". Journal of Chemical Education 51 (8): 553. doi:10.1021/ed051p553. Bibcode: 1974JChEd..51..553H.
- ↑ Brasted, R.; Hirayama, C. (1959). "An Examination of the Absorption Spectra of Some Cobalt(III)-Amine Complexes. Effect of Ligand and Solvents in Absorption". J. Phys. Chem. 63 (6): 780–6. doi:10.1021/j150576a003.
- ↑ Penland, R.; Lane, T.; Quagliano, J. (1956). "Infrared Absorption Spectra of Inorganic Coordination Complexes. VII. Structural Isomerism of Nitro- and Nitritopentaamminecobalt(III) Chlorides". Journal of the American Chemical Society 78 (5): 887–9. doi:10.1021/ja01586a001.
- ↑ Jackson, W.G. (1988). "Oxygen scrambling in pentaamminenitritocobalt(III) revisited". Inorganica Chimica Acta 149: 101–104. doi:10.1016/S0020-1693(00)90574-7.
- ↑ Crawford, B.; Talburt, D.; Johnson, D. (1974). "Effects of Cobalt(III) Complexes on Growth and Metabolism of Escherichia coli". Bioinorganic Chemistry 3 (2): 121–33. doi:10.1016/S0006-3061(00)80035-6. PMID 4613388.
 |

