Chemistry:Patent Blue V
| |

| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| KEGG |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Pharmacology | |
| 1=ATC code }} | V04CX09 (WHO) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
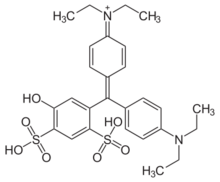
Patent Blue V, also called Food Blue 5, Sulphan Blue, Acid Blue 3, L-Blau 3, C-Blau 20, Patentblau V, Sky Blue, or C.I. 42051, is a sky blue synthetic triphenylmethane dye used as a food coloring.[1] As a food additive, it has E number E131. It is a sodium or calcium salt of [4-(α-(4-diethylaminophenyl)-5-hydroxy- 2,4-disulfophenylmethylidene)-2,5-cyclohexadien-1-ylidene] diethylammonium hydroxide inner salt.
Use as dye
It is not widely used, but in Europe it can be found in Scotch eggs,[citation needed] certain jelly sweets, blue Curaçao, certain gelatin desserts, among others. An important advantage is the very deep color it produces even at low concentration, a disadvantage is that it fades fairly quickly when exposed to light.
In medicine, Patent Blue V is used in lymphangiography and sentinel node biopsy as a dye to color lymph vessels.[2][clarification needed] It is also used in dental disclosing tablets as a stain to show dental plaque on teeth.
The color of the dye is pH-dependent. In aqueous solution, its color will vary from a deep blue in alkaline or weakly acidic medium to a yellow–orange in stronger acidic conditions. It is useful as a pH indicator for the range 0.8–3.0.[3] The structure is also redox-sensitive, changing from a reduced yellow form to an oxidized red form in solution. The reduction potential of around 0.77 volts is similar to that of other triphenylmethane dyes. It is usable as a reversible redox indicator in some analytical methods.[4]
Because of its pH-dependent color, Patent Blue V was included in chemistry sets from Salter Science in the 1970s and 80s under the name Sky Blue.
Regulation
Patent Blue V is banned as a food dye in Australia and US, because health officials in these countries suspect that it may cause allergic reactions, with symptoms ranging from itching[5] and nettle rash to nausea, hypotension, and in rare cases anaphylactic shock; it is therefore not recommended in those countries for children.
References
- ↑ Gessner, Thomas; Mayer, Udo (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_179.
- ↑ Erratum - 44 (4): 649 - The Journal of Nuclear Medicine
- ↑ Sabnis, R. W. (2008). "Patent Blue V". Handbook of Acid–Base Indicators. CRC Press. pp. 295–297. ISBN 9780849382185. https://archive.org/details/handbookacidbase00sabn.
- ↑ Yoe, John H.; Boyd, George R. (1939). "Patent blue V as a pH and redox indicator". Ind. Eng. Chem. Anal. Ed. 11 (9): 492–493. doi:10.1021/ac50137a008.
- ↑ L. Barthelmes, A. Goyal, R.G. Newcombe, F. McNeill, R.E. Mansel, Adverse reactions to patent blue V dye – The NEW START and ALMANAC experience, European Journal of Surgical Oncology (EJSO), Volume 36, Issue 4, April 2010, Pages 399-403, doi:10.1016/j.ejso.2009.10.007.
 |


