Biology:Cardiac muscle cell
| Cardiac muscle cells | |
|---|---|
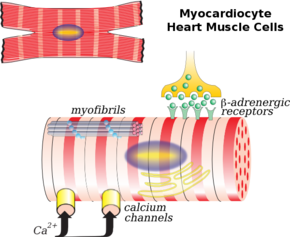 Illustration of a myocardiocyte, including organelles and cell membrane functions | |
| Details | |
| Precursor | Mesoderm |
| System | Cardiovascular system |
| Identifiers | |
| Latin | cardiomyocytus; myocytus cardiacus |
| Anatomical terms of microanatomy | |
Cardiac muscle cells or cardiomyocytes (also known as myocardiocytes[1] or cardiac myocytes[2]) are the muscle cells (myocytes) that make up the cardiac muscle (heart muscle). Each cardiac muscle cell contains myofibrils, which are specialized organelles consisting of long chains of sarcomeres, the fundamental contractile units of muscle cells.
Cardiomyocytes show striations similar to those on skeletal muscle cells. Unlike multinucleated skeletal cells, the majority of cardiomyocytes contain only one nucleus, although they may have as many as four.[3] Cardiomyocytes have a high mitochondrial density, which allows them to produce adenosine triphosphate (ATP) quickly, making them highly resistant to fatigue.
Structure
There are two types of cells within the heart: the cardiomyocytes and the cardiac pacemaker cells. Cardiomyocytes make up the atria (the chambers in which blood enters the heart) and the ventricles (the chambers where blood is collected and pumped out of the heart). These cells must be able to shorten and lengthen their fibers and the fibers must be flexible enough to stretch. These functions are critical to the proper form during the beating of the heart.[4]
Cardiac pacemaker cells carry the impulses that are responsible for the beating of the heart. They are distributed throughout the heart and are responsible for several functions. First, they are responsible for being able to spontaneously generate and send out electrical impulses. They also must be able to receive and respond to electrical impulses from the brain. Lastly, they must be able to transfer electrical impulses from cell to cell.[5]
All of these cells are connected by cellular bridges. Porous junctions called intercalated discs form junctions between the cells. They permit sodium, potassium and calcium to easily diffuse from cell to cell. This makes it easier for depolarization and repolarization in the myocardium. Because of these junctions and bridges the heart muscle is able to act as a single coordinated unit.[6][7]
Cardiomyocytes are about 100μm long and 10-25μm in diameter.[8][9]
Development
Humans are born with a set number of heart muscle cells, or cardiomyocytes, which increase in size as heart grows larger during childhood development. Recent evidence suggests that cardiomyocytes are actually slowly turned over as we age, but that less than 50% of the cardiomyocytes we are born with are replaced during a normal life span.[10] The growth of individual cardiomyocytes not only occurs during normal heart development, it also occurs in response to extensive exercise (athletic heart syndrome), heart disease, or heart muscle injury such as after a myocardial infarction. A healthy adult cardiomyocyte has a cylindrical shape that is approximately 100μm long and 10-25μm in diameter. Cardiomyocyte hypertrophy occurs through sarcomerogenesis, the creation of new sarcomere units in the cell. During heart volume overload, cardiomyocytes grow through eccentric hypertrophy.[8] The cardiomyocytes extend lengthwise but have the same diameter, resulting in ventricular dilation. During heart pressure overload, cardiomyocytes grow through concentric hypertrophy.[8] The cardiomyocytes grow larger in diameter but have the same length, resulting in heart wall thickening.
Function
Depolarization/repolarization cycle
Cardiac action potential consists of two cycles, a rest phase, and an active phase. These two phases are commonly understood as systole and diastole. The rest phase is considered polarized. The resting potential during this phase of the beat separates the ions such as sodium, potassium, and calcium. Myocardial cells possess the property of automaticity or spontaneous depolarization. This is the direct result of a membrane which allows sodium ions to slowly enter the cell until the threshold is reached for depolarization. Calcium ions follow and extend the depolarization even further. Once calcium stops moving inward, potassium ions move out slowly to produce repolarization. The very slow repolarization of the CMC membrane is responsible for the long refractory period.[11][12]
Clinical significance
Myocardial infarction
Myocardial infarction, commonly known as a heart attack, occurs when the arteries supplying blood to the heart muscle become blocked (obstructed). Multiple problems can result in obstruction of coronary arteries, but it is most commonly caused by coronary artery disease, in which the walls of these blood vessels become coated with atherosclerotic plaques made of white blood cells, fat, and other debris. A plaque can rupture suddenly, blocking the coronary artery with debris and clotted blood. With no blood flow, the heart muscle cells die, causing whole portions of cardiac tissue to die. Once these tissues are lost, they cannot be replaced, thus causing permanent damage. Current research indicates, however, that it may be possible to repair damaged cardiac tissue with stem cells.[13][citation needed]
Cardiomyopathy
The cardiomyopathies are a group of diseases characterized by disruptions to cardiac muscle cell growth and / or organization. Presentation can range from asymptomatic to sudden cardiac death. Cardiomyopathy can be caused by genetic, endocrine, environmental, or other factors.
Myocytolysis
Significant damage to cardiac muscle cells is referred to as myocytolysis. It was first described in medical literature by Schlesinger and Reiner in 1955.[14] It is considered a type of cellular necrosis.[14] Two types of myocytolysis have been defined: coagulative and colliquative.[14][15]
See also
- Endocardium
- Epicardium
- Pericardium
- List of human cell types derived from the germ layers
References
- ↑ Wolfgang Kühnel (1 January 2003). Color atlas of cytology, histology, and microscopic anatomy. Thieme. pp. 172–. ISBN 978-3-13-562404-4. https://books.google.com/books?id=wUFAGmVN_aMC&pg=PA172. Retrieved 18 April 2010.
- ↑ Julian Seifter; Austin Ratner; David Sloane (1 October 2005). Concepts in medical physiology. Lippincott Williams & Wilkins. pp. 201–. ISBN 978-0-7817-4489-8. https://books.google.com/books?id=A8H_9S4E0I4C&pg=PA201. Retrieved 18 April 2010.
- ↑ "Aging, cardiac hypertrophy and ischemic cardiomyopathy do not affect the proportion of mononucleated and multinucleated myocytes in the human heart". Journal of Molecular and Cellular Cardiology 28 (7): 1463–77. July 1996. doi:10.1006/jmcc.1996.0137. PMID 8841934.
- ↑ Severs, Nicholas. "The Cardiac Muscle Cell". http://www.ulysse.u-bordeaux.fr/atelier/ikramer/biocell_diffusion/gbb.cel.fa.104.b3/content/pdf/04_11_Cardiac_Myocytes_Severs.pdf.
- ↑ "Anatomy and Physiology of the Heart". http://www.bem.fi/book/06/06.htm.
- ↑ "American Heart Association: How the Heart Works". http://www.heart.org/HEARTORG/Conditions/CongenitalHeartDefects/AboutCongenitalHeartDefects/How-the-Healthy-Heart-Works_UCM_307016_Article.jsp.
- ↑ Martini, Frederic. "The Fundamentals of Anatomy and Physiology: Chapter 10 Cardiac Muscle Tissue". http://cwx.prenhall.com/bookbind/pubbooks/martinidemo/chapter10/medialib/CH10/html/ch10_8.html.
- ↑ 8.0 8.1 8.2 Göktepe, S; Abilez, OJ; Parker, KK; Kuhl, E (2010-08-07). "A multiscale model for eccentric and concentric cardiac growth through sarcomerogenesis". Journal of Theoretical Biology 265 (3): 433–42. doi:10.1016/j.jtbi.2010.04.023. PMID 20447409.
- ↑ Olivetti, G, S; Cigola, E; Maestri, R; Corradi, D; Lagrasta, C; Gambert, SR; Anversa, P (1996-07-28). "Aging, cardiac hypertrophy and ischemic cardiomyopathy do not affect the proportion of mononucleated and multinucleated myocytes in the human heart.". J Mol Cell Cardiol 28 (7): 1463–1477. doi:10.1006/jmcc.1996.0137. PMID 8841934.
- ↑ Bergmann, O.; Bhardwaj, R. D.; Bernard, S.; Zdunek, S.; Barnabe-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K. et al. (3 April 2009). "Evidence for cardiomyocyte renewal in humans". Science 324 (5923): 98–102. doi:10.1126/science.1164680. PMID 19342590. Bibcode: 2009Sci...324...98B.
- ↑ Klabunde, Richard. "Cardiovascular Physiology= Cardiac muscle Concept". http://www.cvphysiology.com/Arrhythmias/A009.htm.
- ↑ "Cells Alive: Pumping Myocytes". http://www.cellsalive.com/myocyte.htm.
- ↑ "Stem Cell Research News". http://stemcellresearchnews.net/News/HowDoesOneMendABrokenHeart.aspx.
- ↑ 14.0 14.1 14.2 Baroldi, Giorgio (2004-01-20) (in en). The Etiopathogenesis of Coronary Heart Disease: A Heretical Theory Based on Morphology, Second Edition. CRC Press. pp. 88. ISBN 9781498712811. https://books.google.com/books?id=7SxiDwAAQBAJ&q=Myocytolysis&pg=PA21.
- ↑ Olsen, E. G. (2012-12-06) (in en). Atlas of Cardiovascular Pathology. Springer Science & Business Media. pp. 48. ISBN 9789400932098. https://books.google.com/books?id=uUP8CAAAQBAJ&q=Myocytolysis+definition&pg=PA48.

