Chemistry:Vindoline
From HandWiki
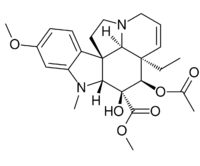
| |
| Names | |
|---|---|
| IUPAC name
Methyl 3β,4β-dihydroxy-16-methoxy-1-methyl-6,7-didehydro-2β,5α,12β,19α-aspidospermidine-3α-carboxylate
| |
| Systematic IUPAC name
Methyl (3aR,3a1R,4R,5S,5aR,10bR)-4-(acetyloxy)-3a-ethyl-5-hydroxy-8-methoxy-6-methyl-3a,3a1,4,5,5a,6,11,12-octahydro-1H-indolizino[8,1-cd]carbazole-5-carboxylate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C25H32N2O6 | |
| Molar mass | 456.539 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Vindoline is a chemical precursor to vinblastine.[1] Vindoline is formed through biosynthesis from Tabersonine.
See also
References
- ↑ Liu, J; Zhu, J; Tang, L; Wen, W; Lv, S; Yu, R (2014). "Enhancement of vindoline and vinblastine production in suspension-cultured cells of Catharanthus roseus by artemisinic acid elicitation". World Journal of Microbiology and Biotechnology 30 (1): 175–80. doi:10.1007/s11274-013-1432-z. PMID 23864440.
 |

