Chemistry:Chlormequat
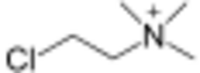
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Chloro-N,N,N-trimethylethan-1-aminium | |
| Other names
Chlorocholine; Chlorcholine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C5H13ClN | |
| Molar mass | 122.62 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chlormequat is an organic compound with the formula ClCH2CH2N(CH3)+3 that is used as a plant growth regulator. It is typically sold as the chloride salt, chlormequat chloride[1] (C5H13Cl2N), a colorless hygroscopic crystalline substance that is soluble in water and ethanol.[2] It is an alkylating agent and a quaternary ammonium salt. Chlormequat is one of the onium-type growth regulators.[3]
Chlormequat has been called the "most important inhibitor of gibberellin biosynthesis."[2] As such, it inhibits cell elongation, resulting in thicker stalks, which are sturdier, facilitating harvesting of cereal crops.[4] It can also be used as an adjuvant for herbicides by retarding their oxidative disposal by plants. This is due to cytochrome P450-inhibition.[3]
Regulation and toxicity
In the United States, chlormequat is classified as a low risk plant growth regulator and it is registered for use on ornamental plants grown in greenhouses, nurseries, and shadehouses.[5] It is not approved for use on crops intended for animal or human consumption.[5]
The -1">50 (rat, oral) is low, approximately 670 mg/kg.[2]
Exposure to high levels of chlormequat has been linked to developmental toxicity in animal models.[6] It also affects reproduction in mammals.[7]
It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.[8]
References
- ↑ Chlormequat chloride (ChemSpider)
- ↑ 2.0 2.1 2.2 Wilhelm Rademacher, Lutz Brahm "Plant Growth Regulators" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2010. doi:10.1002/14356007.a20_415.pub2
- ↑ 3.0 3.1 Rademacher, Wilhelm (2000). "Growth Retardants: Effects on Gibberellin Biosynthesis and Other Metabolic Pathways". Annual Review of Plant Physiology and Plant Molecular Biology (Annual Reviews) 51 (1): 501–531. doi:10.1146/annurev.arplant.51.1.501. ISSN 1040-2519. PMID 15012200.
- ↑ Gowariker, Vasant; Kalyani Paranjape; Sudha Gowariker; V. N. Krishnamurthy (2013). The pesticide encyclopedia. Wallingford: CABI. p. 93. ISBN 978-1780640143.
- ↑ 5.0 5.1 Chlormequat Chloride Reregistration Eligibility Decision for Low Risk Pesticide
- ↑ "Chlormequat". Food and Agricultural Organization of the United Nations. http://www.fao.org/docrep/w8141e/w8141e0k.htm.
- ↑ Sørensen, MT; Danielsen, V (February 2006). "Effects of the plant growth regulator, chlormequat, on mammalian fertility". International Journal of Andrology 29 (1): 129–33. doi:10.1111/j.1365-2605.2005.00629.x. PMID 16466532.
- ↑ 40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities (July 1, 2008 ed.). Government Printing Office. http://edocket.access.gpo.gov/cfr_2008/julqtr/pdf/40cfr355AppA.pdf. Retrieved October 29, 2011.
External links
 |

