Chemistry:Daturaolone
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
(4aR,5R,6aR,6bS,8aR,12aR,14aR,14bR)-5-Hydroxy-4,4,6a,6b,8a,14b-hexamethyl-1,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-octadecahydropicen-3(2H)-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C30H48O2 | |
| Molar mass | 440.712 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
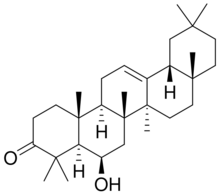
Daturaolone is a pentacyclic oleanane triterpenoid,[1] also known as 3-oxo-6-β-hydroxy-β-amyrin,[2] found in Datura species such as Datura stramonium[3] and Datura innoxia.[4]
History
It was isolated for the first time from Solanum arundo.[5]
Structure
Daturaolone contains five rings with a ketone group and a hydroxyl group, which may be essential for its bioactivities. The structure was deduced through mass spectroscopy and 1H NMR spectroscopy.[6]
Functions
Daturaolone isolated from Datura metel Linnaeus has been found to have anti-fungal and anti-bacterial activities. When tested against bacterial strains such as Klebsiella pneumoniae and S. aureus, daturaolone was shown to inhibit bacterial growth.[7]
See also
- Scopine
- Daturadiol
- Withametelin
References
- ↑ Baig, Muhammad Waleed; Fatima, Humaira; Akhtar, Nosheen; Hussain, Hidayat; Okla, Mohammad K.; Al-Hashimi, Abdulrahman; Al-Qahtani, Wahidah H.; AbdElgawad, Hamada et al. (2021-11-30). "Anti-Inflammatory Potential of Daturaolone from Datura innoxia Mill.: In Silico, In Vitro and In Vivo Studies". Pharmaceuticals 14 (12): 1248. doi:10.3390/ph14121248. ISSN 1424-8247. PMID 34959649.
- ↑ Bawazeer, Saud; Rauf, Abdur; Bawazeer, Sami (2020). "Gastrointestinal Motility, Muscle Relaxation, Antipyretic and Acute Toxicity Screening of Amyrin Type Triterpenoid (Daturaolone) Isolated From Datura metel Linnaeus (Angel's Trumpet) Fruits". Frontiers in Pharmacology 11: 544794. doi:10.3389/fphar.2020.544794. ISSN 1663-9812. PMID 33101017.
- ↑ "[Chemical constituents of Datura stramonium seeds]" (in zh). Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China Journal of Chinese Materia Medica 37 (3): 319–22. February 2012. PMID 22568232.
- ↑ "Triterpenes of Datura innoxia Mill. Structure of daturadiol and daturaolone". The Journal of Organic Chemistry 38 (21): 3685–8. October 1973. doi:10.1021/jo00961a005. PMID 4745874.
- ↑ "Hepato-protective effect of daturaolone isolated from Solanum arundo". Die Pharmazie 51 (8): 593–5. August 1996. PMID 8794471.
- ↑ Grace, M. H.; Saleh, M. M. (August 1996). "Hepato-protective effect of daturaolone isolated from Solanum arundo". Die Pharmazie 51 (8): 593–595. ISSN 0031-7144. PMID 8794471. https://pubmed.ncbi.nlm.nih.gov/8794471/.
- ↑ Bawazeer, Sami; Rauf, Abdur (2021-09-18). "In Vitro Antibacterial and Antifungal Potential of Amyrin-Type Triterpenoid Isolated from Datura metel Linnaeus". BioMed Research International 2021: 1543574. doi:10.1155/2021/1543574. ISSN 2314-6133. PMID 34589544.
 |

