Chemistry:Bisthiosemicarbazone
From HandWiki
In organic chemistry, a bisthiosemicarbazone is a derivative from an elimination reaction between a thiosemicarbazide and a diketone. Their structure is H2NHC(=S)NN=C(R1)−R2−C(R3)=NNHC(=S)NH2. A 'thiosemicarbazone' contains a sulfur atom in lieu of the ketonic oxygen in semicarbazone. Bisthiosemicarbazones are known to have antiviral, antimalarial and anticancer activity,[1] usually mediated through binding to copper or iron in cells. They have also been identified as potential ligands for radioisotope delivery, with selectivity towards hypoxic tissues, particularly in the heart and brain.[2][3][4] When chelated to zinc atoms some bisthiosemicarbazones may have uses as fluorescing agents in optical microscopy.
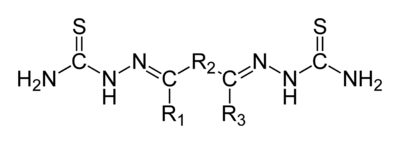
General structural formula of bisthiosemicarbazones
See also
References
- ↑ Palanimuthu, Duraippandi; Shinde, Sridevi Vijay; Somasundaram, Kumaravel; Samuelson, Ashoka G. (14 February 2013). "In Vitro and in Vivo Anticancer Activity of Copper Bis(thiosemicarbazone) Complexes". Journal of Medicinal Chemistry 56 (3): 722–734. doi:10.1021/jm300938r. PMID 23320568.
- ↑ Dearling, Jason L.; Lewis, Jason S.; Mullen, Gregory E.; Welch, Michael J.; Blower, Philip J. (20 April 2014). "Copper bis(thiosemicarbazone) complexes as hypoxia imaging agents: structure-activity relationships". Journal of Biological Inorganic Chemistry 7 (3): 249–259. doi:10.1007/s007750100291. PMID 11935349.
- ↑ Maurer, Richard I.; Blower, Philip J.; Dilworth, Jonathan R.; Reynolds, Christopher A.; Zheng, Yifan; Mullen, Gregory E. D. (March 2002). "Studies on the Mechanism of Hypoxic Selectivity in Copper Bis(Thiosemicarbazone) Radiopharmaceuticals". Journal of Medicinal Chemistry 45 (7): 1420–1431. doi:10.1021/jm0104217. PMID 11906283.
- ↑ Cowley, Andrew R.; Dilworth, Jonathan R.; Donnelly, Paul S.; Labisbal, Elena; Sousa, Antonio (May 2002). "An Unusual Dimeric Structure of a Cu(I) Bis(thiosemicarbazone) Complex: Implications for the Mechanism of Hypoxic Selectivity of the Cu(II) Derivatives". Journal of the American Chemical Society 124 (19): 5270–5271. doi:10.1021/ja012668z. PMID 11996559.
 |

