Chemistry:Meteloidine
| |
| Names | |
|---|---|
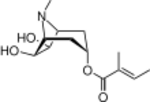
| IUPAC name
[(6R,7S)-6,7-Dihydroxy-8-methyl-8-azabicyclo[3.2.1]octan-3-yl] (E)-2-methylbut-2-enoate
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C13H21NO4 | |
| Molar mass | 255.314 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Meteloidine is an alkaloid found in some Brugmansia and Datura species.[1] Its also found in Erythroxylum australe and is said to be cocaine-like alkaloid.[2]
Occurrence
The first report of the isolation from a natural source of meteloidine was in 1908 by Frank Lee Pyman and William Colebrook Reynolds[3] from the flowering plant Datura metel along Angelate ester and Datura meteloides (now reclassified as Datura innoxia).[4]
Meteloidine is primarily found in solanaceous plants, and in one species of genus Erythroxylum. It has been found in the leaves and flowers of Brugmansia candida,[5] and in the roots of Datura leichhardtii,[6] Brugmansia suaveolens,[7] Anthocercis littorea and Anthocercis viscosa[8] in minor quantities, and in Anthocercis genistoides as its principal alkaloid. Meteloidine has been identified in Erythroxylum australe, which is of chemotaxonomic interest as meteloidine has been found in a number of the Solanacae family, but in only one species in the family Erythroxylaceae.[9]
See also
- Daturadiol
- Serpentine
- Catuabine
References
- ↑ Leete, E; Murrill, J. B (1967). "Biosynthesis of the tiglic acid moiety of meteloidine in Datura meteloides". Tetrahedron Letters 18: 1727–30. doi:10.1016/s0040-4039(00)90710-x. PMID 6045963.
- ↑ "Erowid Psychoactive Vaults : Australian Natural Highs FAQ". https://www.erowid.org/psychoactives/faqs/faq_natural_high_australia.shtml#COCAINE.
- ↑ Pyman, Frank Lee (1 January 1908). "Meteloidine : A New Solanaceous Alkaloid". Journal of the Chemical Society 93 (93): 2077–2081. doi:10.1039/CT9089302077. https://pubs.rsc.org/en/content/articlepdf/1908/ct/ct9089302077. Retrieved 11 September 2021.
- ↑ Barclay, Arthur S (January 16, 1959). "New Considerations in an Old Genus: Datura". Botanical Museum Leaflets, Harvard University 18 (6): 245–272. doi:10.5962/p.168515.
- ↑ Griffin, W J (1966). "Alkaloids in Datura, Section Brugmansia". Planta Medica 14 (4): 468–474. doi:10.1055/s-0028-1100075. https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0028-1100075. Retrieved 11 September 2021.
- ↑ Evans, W C (1962). "Studies on Datura Leichhardtii Muell. Ex Benth.". Journal of Pharmacy and Pharmacology 14 (1): 107T–110T. doi:10.1111/j.2042-7158.1962.tb10542.x. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.2042-7158.1962.tb10542.x?__cf_chl_jschl_tk__=pmd_SJ0tRwXVzMp1DpWTN9MnMr1p9vUNQoOydtCHuJmGgzc-1631382946-0-gqNtZGzNAhCjcnBszQxR.
- ↑ Evans, W C (November 1972). "Alkaloids of Datura suaveolens". Phytochemistry 11 (11): 3293–3298. doi:10.1016/s0031-9422(00)86392-x. https://www.sciencedirect.com/science/article/pii/S003194220086392X. Retrieved 11 September 2021.
- ↑ El Imam, Y (1984). "Tropane Alkaloids of Species of Anthocercis, Cyphanthera and Crenidium". Planta Medica 50 (1): 86–87. doi:10.1055/s-2007-969628. PMID 17340258. https://dx.doi.org/10.1055/s-2007-969628. Retrieved 11 September 2021.
- ↑ Johns, S R (1967). "Meteloidine from Erythroxylum australe F. Muell". Australian Journal of Chemistry 20 (6): 1301–1302. doi:10.1071/CH9671301. https://www.publish.csiro.au/ch/pdf/CH9671301.
 |

