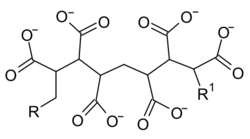
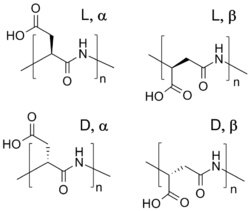
Chemistry:Polycarboxylates
Polycarboxylates are linear polymers with a high molecular mass (Mr ≤ 100 000) and with many carboxylate groups. They are polymers of acrylic acid or copolymers of acrylic acid and maleic acid. The polymer is used as the sodium salt (see: sodium polyacrylate).[1]
Use
Polycarboxylates are used as builders in detergents.[2] Their high chelating power, even at low concentrations, reduces deposits on the laundry and inhibits the crystal growth of calcite.
Polycarboxylate ethers (PCE) are used as superplasticizers in concrete production.[3]
Safety
Polycarboxylates are poorly biodegradable but have a low ecotoxicity. In the sewage treatment plant, the polymer remains largely in the sludge and is separated from the wastewater.
Polyamino acids like polyaspartic acid and polyglutamic acid have better biodegradability but lower chelating performance than polyacrylates. They are also less stable towards heat and alkali. Since they contain nitrogen, they contribute to eutrophication.[4]
References
- ↑ Entry on Polycarboxylate. at: Römpp Online. Georg Thieme Verlag, retrieved 17. August 2016.
- ↑ Polycarboxylates, The Soap and Detergent Association, 1996, http://www.aciscience.org/docs/Polycarboxylates.pdf, retrieved 2013-09-23
- ↑ Susanne Palecki (2006), Hochleistungsbeton unter Frost-Tau-Wechselbelastung: Schädigungs- und Transportmechanismen, p. 20, ISBN 978-3-86537-725-8, https://books.google.com/books?id=EDB2lOrNmQMC
- ↑ Yangxin Yu; Jin Zhao; Andrew E. Bayly (2008), "Development of Surfactants and Builders in Detergent Formulations", Chinese Journal of Chemical Engineering 16 (4): 517–527, doi:10.1016/S1004-9541(08)60115-9, http://www.cjche.com.cn/CN/Y2008/V16/I4/517
 |