Chemistry:Stearoyl-CoA
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
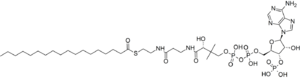
3′-O-Phosphonoadenosine 5′-{(3R)-3-hydroxy-2,2-dimethyl-4-[(3-{[2-(octadecanoylsulfanyl)ethyl]amino}-3-oxopropyl)amino]-4-oxobutyl dihydrogen diphosphate}
| |
| Systematic IUPAC name
O1-{[(2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methyl} O3-{(3R)-3-hydroxy-2,2-dimethyl-4-[(3-{[2-(octadecanoylsulfanyl)ethyl]amino}-3-oxopropyl)amino]-4-oxobutyl} dihydrogen diphosphate | |
| Other names
S-Stearoylcoenzyme A, Stearyl-CoA, Octadecanoyl-coenzyme A, Octadecanoyl-CoA, stearyl coenzyme A
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C39H70N7O17P3S | |
| Molar mass | 1034.00 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Stearoyl-CoA is a coenzyme involved in the metabolism of fatty acids.[1] Stearoyl-CoA is an 18-carbon long fatty acyl-CoA chain that participates in an unsaturation reaction. The reaction is catalyzed by the enzyme stearoyl-CoA desaturase, which is located in the endoplasmic reticulum.[2] It forms a cis-double bond between the ninth and tenth carbons within the chain to form the product oleoyl-CoA.[3]
References
- ↑ Ntambi, J. M. (2002). "Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity". Proceedings of the National Academy of Sciences 99 (17): 11482–11486. doi:10.1073/pnas.132384699. ISSN 0027-8424. PMID 12177411. Bibcode: 2002PNAS...9911482N.
- ↑ Ntambi, James (2013). Stearoyl-CoA Desaturase Genes in Lipid Metabolism. Springer. ISBN 978-1-4614-7969-7.
- ↑ Igal, R. Ariel (December 2016). "Stearoyl CoA desaturase-1: New insights into a central regulator of cancer metabolism". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 1861 (12): 1865–1880. doi:10.1016/j.bbalip.2016.09.009. PMID 27639967.
Bibliography
- Miyazaki, M. (2000). "The Biosynthesis of Hepatic Cholesterol Esters and Triglycerides Is Impaired in Mice with a Disruption of the Gene for Stearoyl-CoA Desaturase 1". Journal of Biological Chemistry 275 (39): 30132–30138. doi:10.1074/jbc.M005488200. PMID 10899171.
 |

