Medicine:Dilation and curettage
Template:Infobox abortion method
Dilation (or dilatation) and curettage (D&C) refers to the dilation (widening or opening) of the cervix and surgical removal of part of the lining of the uterus or contents of the uterus by scraping and scooping (curettage). It is a gynecologic procedure used for diagnostic and therapeutic purposes, and is the most commonly used method for first-trimester miscarriage or abortion.[1][2][3][4]
D&C normally refers to a procedure involving a curette, also called sharp curettage.[2] However, some sources use the term D&C to refer to any procedure that involves the processes of dilation and removal of uterine contents, which includes the more common suction curettage procedures of manual and electric vacuum aspiration.[5]
Clinical uses
D&Cs may be performed in pregnant and non-pregnant patients, for different clinical indications.
During pregnancy or postpartum
A D&C may be performed early in pregnancy to remove pregnancy tissue, either in the case of a non-viable pregnancy, such as a missed or incomplete miscarriage, or an undesired pregnancy, as in a surgical abortion.[6] Medical management of miscarriage and medical abortion using drugs such as misoprostol and mifepristone are safe, non-invasive and potentially less expensive alternatives to D&C.
Because medication-based non-invasive methods of abortion now exist, dilation and curettage has been declining as a method of abortion, although suction curettage is still the most common method used for termination of a first-trimester pregnancy.[7][8] The World Health Organization recommends D&C with a sharp curette as a method of surgical abortion only when manual vacuum aspiration with a suction curette is unavailable.[9]
For patients who have recently given birth, a D&C may be indicated to remove retained placental tissue that does not pass spontaneously or for postpartum hemorrhage.[10]
Non-pregnant patients
D&Cs for non-pregnant patients are commonly performed for the diagnosis of gynecological conditions leading to abnormal uterine bleeding;[11] to remove the excess uterine lining in women who have conditions such as polycystic ovary syndrome;[12] to remove tissue in the uterus that may be causing abnormal uterine bleeding, such as endometrial polyps or uterine fibroids;[3][2] or to diagnose the cause of post-menopausal bleeding, such as in the case of endometrial cancer.
Hysteroscopy is a valid alternative or addition to D&C for many surgical indications, from diagnosis of uterine pathology to the removal of fibroids and even retained products of conception. It allows direct visualization of the inside of the uterus and may allow targeted sampling and removal of tissue inside the uterus.[13]
Procedure
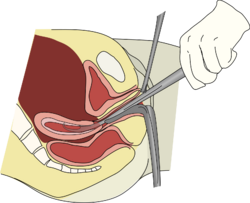
Depending on the anticipated duration and difficulty expected with the procedure, as well as the clinical indication and patient preferences, a D&C may be performed with local anesthesia, moderate sedation, deep sedation, or general anesthesia.[14] The first step in a D&C is to place a speculum in the vagina so as to see the cervix. Often, a tenaculum is placed to steady the cervix. Next, the provider will dilate the cervix. This can be done with Hegar or similar dilators.[6] The amount of dilation depends on the amount of tissue to be removed as well as the size of the instruments to be used. After sufficient dilation, a curette, a metal rod with a handle on one end and a loop on the other, is then inserted into the uterus through the dilated cervix. The curette is used to gently scrape the lining of the uterus and remove the tissue in the uterus. If a suction curette is used, as in a vacuum aspiration, a plastic tubular curette will be introduced into the uterus and connected to suction to remove all tissue in the uterus. This tissue is examined for completeness (in the case of abortion or miscarriage treatment) or by pathology for abnormalities (in the case of treatment for abnormal bleeding).[2]
Complications
The most common complications associated with D&C are infection, bleeding, or damage to nearby organs, including through uterine perforation.[15] Aside from the surgery itself, complications related to anesthesia administration may also occur.
Infection is uncommon after D&C for a non-pregnant patient, and society practice guidelines do not recommend routine prophylactic antibiotics to patients.[16] However, for curettage of a pregnant patient, the risk of infection is higher, and patients should receive antibiotics that cover the bacteria commonly found in the vagina and gastrointestinal tract; doxycycline is a common recommendation, though azithromycin may also be used.[16]
Another risk of D&C is uterine perforation. The highest rate of uterine perforation appears to be in the setting of postpartum hemorrhage (5.1%) compared with a lower rate in diagnostic curettage in non-pregnant patients (0.3% in the premenopausal patient and 2.6% in the postmenopausal patient).[17] Perforation may cause excessive bleeding or damage to organs outside the uterus. If the provider is concerned about ongoing bleeding or the possibility of injury to organs outside the uterus, a laparoscopy may be done to verify that there has been no undiagnosed injury.
Another potential risk is Asherman’s syndrome, a condition where intrauterine adhesions lead to subfertility, amenorrhea, or recurrent pregnancy loss. Although older studies[18][19][20] described a high (25-30%) risk of developing this condition after dilation and curettage for treatment of miscarriage, these procedures were likely done using sharp curettage, which is no longer routinely performed in modern miscarriage and abortion care. Newer studies[21][22] reflect the common technique of suction curettage and demonstrate a much lower risk of Asherman’s syndrome, with incidence in large prospective trials ranging from 0.7-1.6%. A history of multiple (>3) procedures[21] and sharp curettage[22] were identified as risk factors for developing clinical Asherman’s syndrome. A systematic review in 2013 concluded that recurrent miscarriage treated with D&C is the main risk factors for intrauterine adhesions.[23] There are currently no studies linking asymptomatic intrauterine adhesions and long-term reproductive outcomes, and similar pregnancy outcomes have been found after miscarriage regardless of whether surgical treatment, medication management, or conservative management (i.e. watchful waiting) was chosen.[23]
See also
- Dilation and evacuation
- Menstrual extraction
- Vacuum aspiration
References
- ↑ Pazol, Karen; Creanga, Andreea A.; Burley, Kim D. et al. (November 29, 2013). "Abortion Surveillance – United States, 2010". Surveillance Summaries (Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control) 62 (ss08): 1–44. PMID 24280963. https://www.cdc.gov/mmwr/preview/mmwrhtml/ss6208a1.htm?s_cid=ss6208a1_w#tab11. Retrieved 14 January 2014.
- ↑ 2.0 2.1 2.2 2.3 "Dilation and sharp curettage (D&C) for abortion". 2004-10-07. http://women.webmd.com/dilation-and-sharp-curettage-dc-for-abortion.
- ↑ 3.0 3.1 Hayden, Merrill (2006-02-22). "Dilation and curettage (D&C) for dysfunctional uterine bleeding". http://www.webmd.com/sexual-conditions/Dilation-and-curettage-DC-for-dysfunctional-uterine-bleeding.
- ↑ Nissl, Jan (2005-01-18). "Dilation and curettage (D&C) for bleeding during menopause". http://www.webmd.com/menopause/Dilation-and-curettage-DC-for-bleeding-during-menopause.
- ↑ "What Every Pregnant Woman Needs to Know About Pregnancy Loss and Neonatal Death". WebMD. 2004-10-07. http://www.webmd.com/sexual-conditions/pregnancy-loss-neonatal-death?page=3.
- ↑ 6.0 6.1 "ACOG: FAQ: Dilation and Curettage". https://www.acog.org/Patients/FAQs/Dilation-and-Curettage.
- ↑ "Minor surgical procedure common in O&G associated with increased risk of preterm delivery". European Society of Human Reproduction and Embryology. 16 June 2015. https://www.eurekalert.org/pub_releases/2015-06/esoh-msp061015.php.
- ↑ "Induced Abortion in the United States". Guttmacher Institute. September 2019. https://www.guttmacher.org/fact-sheet/induced-abortion-united-states.
- ↑ Managing complications in pregnancy and childbirth: a guide for midwives and doctors. World Health Organization, UNICEF, United Nations Population Fund. 2017. p. P-71. ISBN 9789241565493.
- ↑ "Evacuating retained products of conception in the setting of an ultrasound unit". Fertil. Steril. 91 (4 Suppl): 1586–1588. 2009. doi:10.1016/j.fertnstert.2008.10.032. PMID 19064261.
- ↑ "Endometrial polyps: prevalence, detection, and malignant potential in women with abnormal uterine bleeding". Eur J Gynaecol Oncol. 21 (2): 180–183. 2000. PMID 10843481.
- ↑ "Dilation and Curettage (D&C)". Practice Builders & Health Central Women's Care, PA. 2016. http://www.phyllisgeemd.com/Home/PatientEducation/tabid/10903/ctl/View/mid/18015/Default.aspx?ContentPubID=445.
- ↑ "Hysteroscopy". https://my.clevelandclinic.org/health/treatments/10142-hysteroscopy.
- ↑ Allen, Rebecca H.; Singh, Rameet (2018-06-01). "Society of Family Planning clinical guidelines pain control in surgical abortion part 1 – local anesthesia and minimal sedation" (in en). Contraception 97 (6): 471–477. doi:10.1016/j.contraception.2018.01.014. ISSN 0010-7824. PMID 29407363. https://www.contraceptionjournal.org/article/S0010-7824(18)30036-2/abstract.
- ↑ "Dilation and curettage (D&C)". Mayo Clinic. https://www.mayoclinic.org/tests-procedures/dilation-and-curettage/about/pac-20384910.
- ↑ 16.0 16.1 "ACOG Practice Bulletin No. 195: Prevention of Infection After Gynecologic Procedures". Obstetrics & Gynecology (American College of Obstetricians Gynecologists' Committee on Practice Bulletins – Gynecology) 131 (6): e172–e189. June 2018. doi:10.1097/AOG.0000000000002670. ISSN 0029-7844. PMID 29794678.
- ↑ Hefler, Lukas; Lemach, Andrea; Seebacher, Veronika et al. (June 2009). "The Intraoperative Complication Rate of Nonobstetric Dilation and Curettage". Obstetrics & Gynecology 113 (6): 1268–1271. doi:10.1097/AOG.0b013e3181a66f91. ISSN 0029-7844. PMID 19461421.
- ↑ "Incidence of post-abortion intra-uterine adhesions evaluated by hysteroscopy – a prospective study". Hum. Reprod. 8 (3): 442–444. 1993. doi:10.1093/oxfordjournals.humrep.a138068. PMID 8473464.
- ↑ "Intra-uterine adhesions: an updated appraisal". Fertility and Sterility 37 (5): 593–610. 1982. doi:10.1016/S0015-0282(16)46268-0. PMID 6281085.
- ↑ "Intrauterine adhesions and fertility outcome:how to optimize success?". Current Opinion in Obstetrics and Gynecology 19 (3): 207–214. 2007. doi:10.1097/GCO.0b013e32814a6473. PMID 17495635.
- ↑ 21.0 21.1 Sevinç, Fahrünnisa; Oskovi-Kaplan, Z. Asli; Çelen, Şevki; Ozturk Atan, Deniz; Topçu, Hasan Onur (2021). "Identifying the risk factors and incidence of Asherman Syndrome in women with p ost-abortion uterine curettage" (in en). Journal of Obstetrics and Gynaecology Research 47 (4): 1549–1555. doi:10.1111/jog.14667. ISSN 1341-8076. PMID 33462894. https://onlinelibrary.wiley.com/doi/10.1111/jog.14667.
- ↑ 22.0 22.1 Gilman Barber, Ashley R.; Rhone, Stephanie A.; Fluker, Margo R. (2014). "Curettage and Asherman's Syndrome—Lessons to (Re-) Learn?". Journal of Obstetrics and Gynaecology Canada 36 (11): 997–1001. doi:10.1016/s1701-2163(15)30413-8. ISSN 1701-2163. PMID 25574677.
- ↑ 23.0 23.1 "Systematic review and meta-analysis of intrauterine adhesions after miscarriage: Prevalence, risk factors and long-term reproductive outcome". Human Reproduction Update 20 (2): 262–278. 2013. doi:10.1093/humupd/dmt045. PMID 24082042. https://academic.oup.com/humupd/article-pdf/20/2/262/17158384/dmt045.pdf.
External links
- Dilation and curettage (D&C) at Mayo Clinic
 |