Physics:Grain boundary diffusion coefficient
The grain boundary diffusion coefficient is the diffusion coefficient of a diffusant along a grain boundary in a polycrystalline solid.[1] It is a physical constant denoted [math]\displaystyle{ D_b }[/math], and it is important in understanding how grain boundaries affect atomic diffusivity. Grain boundary diffusion is a commonly observed route for solute migration in polycrystalline materials. It dominates the effective diffusion rate at lower temperatures in metals and metal alloys. Take the apparent self-diffusion coefficient for single-crystal and polycrystal silver, for example. At high temperatures, the coefficient [math]\displaystyle{ D_b }[/math] is the same in both types of samples. However, at temperatures below 700 °C, the values of [math]\displaystyle{ D_b }[/math] with polycrystal silver consistently lie above the values of [math]\displaystyle{ D_b }[/math] with a single crystal.[2]
Measurement
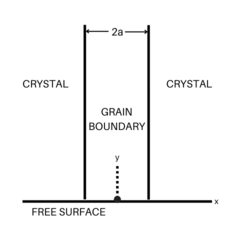
The general way to measure grain boundary diffusion coefficients was suggested by Fisher.[3] In the Fisher model, a grain boundary is represented as a thin layer of high-diffusivity uniform and isotropic slab embedded in a low-diffusivity isotropic crystal. Suppose that the thickness of the slab is [math]\displaystyle{ \delta }[/math], the length is [math]\displaystyle{ y }[/math], and the depth is a unit length, the diffusion process can be described as the following formula. The first equation represents diffusion in the volume, while the second shows diffusion along the grain boundary, respectively.
[math]\displaystyle{ \frac{\partial c}{\partial t}=D\left({\partial^2 c\over\partial x^2}+{\partial^2 c\over\partial y^2}\right) }[/math] where [math]\displaystyle{ |x|\gt \delta/2 }[/math]
[math]\displaystyle{ \frac{\partial c_b}{\partial t}=D_b\left({\partial^2 c_b\over\partial y^2}\right)+\frac{2D}{\delta}\left(\frac{\partial c}{\partial x}\right)_{x=\delta/2} }[/math]
where [math]\displaystyle{ c(x, y, t) }[/math] is the volume concentration of the diffusing atoms and [math]\displaystyle{ c_b(y, t) }[/math] is their concentration in the grain boundary.
To solve the equation, Whipple introduced an exact analytical solution. He assumed a constant surface composition, and used a Fourier–Laplace transform to obtain a solution in integral form.[4] The diffusion profile therefore can be depicted by the following equation.
[math]\displaystyle{ (dln\bar{c}/dy^{6/5})^{5/3}=0.66(D_1/t)^{1/2}(1/D_b\delta) }[/math]
To further determine [math]\displaystyle{ D_b }[/math], two common methods were used. The first is used for accurate determination of [math]\displaystyle{ D_b \delta }[/math]. The second technique is useful for comparing the relative [math]\displaystyle{ D_b \delta }[/math] of different boundaries.
- Method 1: Suppose the slab was cut into a series of thin slices parallel to the sample surface, we measure the distribution of in-diffused solute in the slices, [math]\displaystyle{ c(y) }[/math]. Then we used the above formula that developed by Whipple to get [math]\displaystyle{ D_b \delta }[/math].
- Method 2: To compare the length of penetration of a given concentration at the boundary [math]\displaystyle{ \ \Delta y }[/math] with the length of lattice penetration from the surface far from the boundary.
References
- ↑ P. Heitjans, J. Karger, Ed, “Diffusion in condensed matter: Methods, Materials, Models,” 2nd edition, Birkhauser, 2005, pp. 1-965.
- ↑ Shewmon, Paul (2016). Diffusion in Solids. doi:10.1007/978-3-319-48206-4. ISBN 978-3-319-48564-5. Bibcode: 2016diso.book.....S. http://dx.doi.org/10.1007/978-3-319-48206-4.
- ↑ Fisher, J. C. (January 1951). "Calculation of Diffusion Penetration Curves for Surface and Grain Boundary Diffusion" (in en). Journal of Applied Physics 22 (1): 74–77. doi:10.1063/1.1699825. ISSN 0021-8979. Bibcode: 1951JAP....22...74F. http://aip.scitation.org/doi/10.1063/1.1699825.
- ↑ Whipple, R.T.P. (1954-12-01). "CXXXVIII. Concentration contours in grain boundary diffusion". The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science 45 (371): 1225–1236. doi:10.1080/14786441208561131. ISSN 1941-5982. https://doi.org/10.1080/14786441208561131.
See also
- Kirkendall effect
- Phase transformations in solids
- Mass diffusivity
 |

