Physics:Interferometric scattering microscopy
Interferometric scattering microscopy (iSCAT) refers to a class of methods that detect and image a subwavelength object by interfering the light scattered by it with a reference light field. The underlying physics is shared by other conventional interferometric methods such as phase contrast or differential interference contrast, or reflection interference microscopy. The key feature of iSCAT is the detection of elastic scattering from subwavelength particles, also known as Rayleigh scattering, in addition to reflected or transmission signals from supra-wavelength objects. Typically, the challenge is the detection of tiny signals on top of large and complex, speckle-like backgrounds. iSCAT has been used to investigate nanoparticles such as viruses, proteins, lipid vesicles, DNA, exosomes, metal nanoparticles, semiconductor quantum dots, charge carriers and single organic molecules without the need for a fluorescent label.
Historical background
The principle of interference plays a central role in many imaging methods, including bright-field imaging because it can be described as the interference between the illumination field and the one that has interacted with the object, i.e. through extinction. In fact, even microscopy based on the interference with an external light field is more than one hundred years old.
The first iSCAT-type of measurements were performed in the biophysics community in the 1990s.[1] A systematic development of the method for the detection of nano-objects started in the early 2000s as a general effort to explore fluorescence-free options for studying single molecules and nano-objects.[2] In particular, gold nanoparticles down to a size of 5 nm were imaged via the interference of their scattered light with a reflected beam from the cover-slip supporting them. Using a supercontinuum laser additionally allowed for recording the particles' plasmon spectra.[2] The early measurements were limited by residual speckle-like background. A new approach to background subtraction and the acronym iSCAT were introduced in 2009.[3] Since then, a series of important works has been reported by various groups.[4][5][6][7] Notably, further innovations in background and noise suppression have led to the development of new quantification methods such as mass photometry (originally introduced as iSCAMS), in which ultrasensitive and accurate interferometric detection is converted into a quantitative means for measuring the molecular mass of single biomolecules.[8]
Theoretical background
When a reference light is superposed with an object's scattered light, the intensity at the detector can be described by,[2][7]
[math]\displaystyle{ I_{det} \propto |\overline{E_r} + \overline{E_s}|^2 = I_r + I_s + 2 E_r E_s \cos \phi }[/math]
where [math]\displaystyle{ \overline{E_r} = E_r e^{i \phi_r} }[/math] and [math]\displaystyle{ \overline{E_s} = E_s e^{i \phi_s} }[/math] are the complex electric fields of the reference and scattered light. The resulting terms are the intensity of the reference beam [math]\displaystyle{ (I_r = |\overline{E_r}|^2) }[/math], the pure scattered light from the object [math]\displaystyle{ (I_s = |\overline{E_s}|^2) }[/math], and the cross-term [math]\displaystyle{ (2 E_r E_s \cos \phi) }[/math] which contains a phase [math]\displaystyle{ \phi = \phi_r - \phi_s }[/math]. This phase comprises a Gouy phase component from the variations of the wave vectors, a scattering phase component from the material properties of the object, and a sinusoidally modulating phase component which depends on the position of the particle.
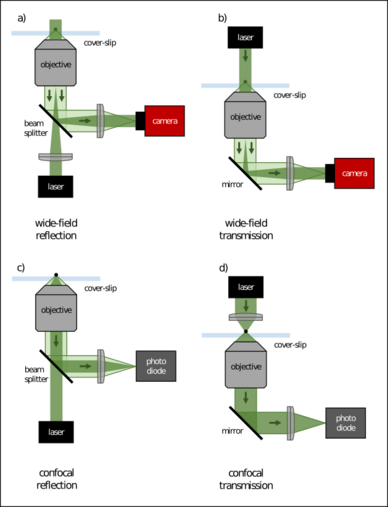
In general, the reference beam can take a different path than the scattered light within the optical setup, as long as they are coherent and interfere on the detector. However, the technique becomes simpler and more stable if both beams share the same optical path. Therefore, the reflected light off the cover-slip or the transmitted beam through the sample is typically used as the reference. For the interference to occur, it is necessary that both light waves (scattered light and reference light) are coherent. Interestingly, a light source with a large coherence length on the order of meters or more (like in modern narrow-band laser systems) is typically not needed. In the most common iSCAT realization schemes where the reflected light of a cover-slip is used as a reference and the scattering particle is not more than a few hundreds of nanometers above the glass, even "incoherent" light, e.g. from LEDs, can be used.[9]
Applications
iSCAT has been used in multiple applications. These can be grouped roughly as:
Label-free imaging
- Microtubules[1][10]
- Live-cell intracellular organelles[11]
- Lipid nano/microdomains[12]
- Single virus assembly[13]
- Time-dependent iSCAT (StroboSCAT)[14]
Single particle tracking
- Single virus tracking in vitro[3]
- Single virus tracking during early-stage infection in cells[15]
- Microsecond single particle tracking on a living cell membrane[16]
- Motor protein tracking[17][18]
Label-free single molecule detection, imaging, tracking and quantification
- Single molecule detection by absorption[19]
- Single protein sensing[20][21]
- Single protein tracking[22]
- Mass photometry[8]
Ion tracking
- Single ions entering/leaving battery cathode[23]
References
- ↑ 1.0 1.1 AMOS, L. A.; AMOS, W. B. (1991-01-01). "The bending of sliding microtubules imaged by confocal light microscopy and negative stain electron microscopy". Journal of Cell Science 1991 (Supplement 14): 95–101. doi:10.1242/jcs.1991.supplement_14.20. ISSN 0021-9533. PMID 1715872.
- ↑ 2.0 2.1 2.2 Lindfors, K.; Kalkbrenner, T.; Stoller, P.; Sandoghdar, V. (July 2004). "Detection and Spectroscopy of Gold Nanoparticles Using Supercontinuum White Light Confocal Microscopy". Physical Review Letters 93 (3): 037401. doi:10.1103/physrevlett.93.037401. ISSN 0031-9007. PMID 15323866. Bibcode: 2004PhRvL..93c7401L.
- ↑ 3.0 3.1 Kukura, Philipp; Ewers, Helge; Müller, Christian; Renn, Alois; Helenius, Ari; Sandoghdar, Vahid (2009-11-01). "High-speed nanoscopic tracking of the position and orientation of a single virus". Nature Methods 6 (12): 923–927. doi:10.1038/nmeth.1395. ISSN 1548-7091. PMID 19881510.
- ↑ Hsieh, Chia-Lung (September 2018). "Label-free, ultrasensitive, ultrahigh-speed scattering-based interferometric imaging". Optics Communications 422: 69–74. doi:10.1016/j.optcom.2018.02.058. ISSN 0030-4018. Bibcode: 2018OptCo.422...69H.
- ↑ Label-free super-resolution microscopy. Astratov, Vasily. Cham. 31 August 2019. ISBN 978-3-030-21722-8. OCLC 1119720519.
- ↑ Young, Gavin; Kukura, Philipp (2019-06-14). "Interferometric Scattering Microscopy". Annual Review of Physical Chemistry 70 (1): 301–322. doi:10.1146/annurev-physchem-050317-021247. ISSN 0066-426X. PMID 30978297. Bibcode: 2019ARPC...70..301Y.
- ↑ 7.0 7.1 Taylor, Richard W.; Sandoghdar, Vahid (2019-07-17). "Interferometric Scattering Microscopy: Seeing Single Nanoparticles and Molecules via Rayleigh Scattering". Nano Letters 19 (8): 4827–4835. doi:10.1021/acs.nanolett.9b01822. ISSN 1530-6984. PMID 31314539. Bibcode: 2019NanoL..19.4827T.
- ↑ 8.0 8.1 Young, Gavin; Hundt, Nikolas; Cole, Daniel; Fineberg, Adam; Andrecka, Joanna; Tyler, Andrew; Olerinyova, Anna; Ansari, Ayla et al. (2018-04-27). "Quantitative mass imaging of single biological macromolecules" (in en). Science 360 (6387): 423–427. doi:10.1126/science.aar5839. ISSN 0036-8075. PMID 29700264. Bibcode: 2018Sci...360..423Y.
- ↑ Daaboul, G.G.; Vedula, R.S.; Ahn, S.; Lopez, C.A.; Reddington, A.; Ozkumur, E.; Ünlü, M.S. (January 2011). "LED-based Interferometric Reflectance Imaging Sensor for quantitative dynamic monitoring of biomolecular interactions". Biosensors and Bioelectronics 26 (5): 2221–2227. doi:10.1016/j.bios.2010.09.038. ISSN 0956-5663. PMID 20980139.
- ↑ Vala, Milan; Bujak, Łukasz; García Marín, Antonio; Holanová, Kristýna; Henrichs, Verena; Braun, Marcus; Lánský, Zdeněk; Piliarik, Marek (2021-01-25). "Nanoscopic Structural Fluctuations of Disassembling Microtubules Revealed by Label‐Free Super‐Resolution Microscopy" (in en). Small Methods 5 (4): 2000985. doi:10.1002/smtd.202000985. ISSN 2366-9608. PMID 34927839.
- ↑ Küppers, Michelle; Albrecht, David; Kashkanova, Anna D.; Lühr, Jennifer; Sandoghdar, Vahid (7 April 2023). "Confocal interferometric scattering microscopy reveals 3D nanoscopic structure and dynamics in live cells" (in en). Nature Communications 14 (1): 1962. doi:10.1038/s41467-023-37497-7. ISSN 2041-1723. PMID 37029107. Bibcode: 2023NatCo..14.1962K.
- ↑ de Wit, Gabrielle; Danial, John S. H.; Kukura, Philipp; Wallace, Mark I. (2015-09-23). "Dynamic label-free imaging of lipid nanodomains". Proceedings of the National Academy of Sciences 112 (40): 12299–12303. doi:10.1073/pnas.1508483112. ISSN 0027-8424. PMID 26401022. Bibcode: 2015PNAS..11212299D.
- ↑ Garmann, Rees F.; Goldfain, Aaron M.; Manoharan, Vinothan N. (2018). "Measurements of the self-assembly kinetics of individual viral capsids around their RNA genome". Proceedings of the National Academy of Sciences 116 (45): 22485–22490. doi:10.1073/pnas.1909223116. PMID 31570619.
- ↑ Penwell, Samuel B.; Ginsberg, Lucas D. S.; Noriega, Rodrigo; Ginsberg, Naomi S. (2017-09-18). "Resolving ultrafast exciton migration in organic solids at the nanoscale". Nature Materials 16 (11): 1136–1141. doi:10.1038/nmat4975. ISSN 1476-1122. PMID 28920937. Bibcode: 2017NatMa..16.1136P.
- ↑ Huang, Yi-Fan; Zhuo, Guan-Yu; Chou, Chun-Yu; Lin, Cheng-Hao; Chang, Wen; Hsieh, Chia-Lung (2017-01-13). "Coherent Brightfield Microscopy Provides the Spatiotemporal Resolution To Study Early Stage Viral Infection in Live Cells". ACS Nano 11 (3): 2575–2585. doi:10.1021/acsnano.6b05601. ISSN 1936-0851. PMID 28067508.
- ↑ Taylor, Richard W.; Mahmoodabadi, Reza Gholami; Rauschenberger, Verena; Giessl, Andreas; Schambony, Alexandra; Sandoghdar, Vahid (July 2019). "Interferometric scattering microscopy reveals microsecond nanoscopic protein motion on a live cell membrane" (in en). Nature Photonics 13 (7): 480–487. doi:10.1038/s41566-019-0414-6. ISSN 1749-4893. Bibcode: 2019NaPho..13..480T. https://www.nature.com/articles/s41566-019-0414-6.
- ↑ Andrecka, J.; Takagi, Y.; Mickolajczyk, K.J.; Lippert, L.G.; Sellers, J.R.; Hancock, W.O.; Goldman, Y.E.; Kukura, P. (2016), "Interferometric Scattering Microscopy for the Study of Molecular Motors", Single-Molecule Enzymology: Fluorescence-Based and High-Throughput Methods (Elsevier) 581: pp. 517–539, doi:10.1016/bs.mie.2016.08.016, ISBN 978-0-12-809267-5, PMID 27793291
- ↑ Bujak, Łukasz; Holanová, Kristýna; García Marín, Antonio; Henrichs, Verena; Barvík, Ivan; Braun, Marcus; Lánský, Zdeněk; Piliarik, Marek (2021-08-16). "Fast Leaps between Millisecond Confinements Govern Ase1 Diffusion along Microtubules" (in en). Small Methods 5 (10): 2100370. doi:10.1002/smtd.202100370. ISSN 2366-9608. PMID 34927934. https://onlinelibrary.wiley.com/doi/10.1002/smtd.202100370.
- ↑ Kukura, Philipp; Celebrano, Michele; Renn, Alois; Sandoghdar, Vahid (2010-11-11). "Single-Molecule Sensitivity in Optical Absorption at Room Temperature". The Journal of Physical Chemistry Letters 1 (23): 3323–3327. doi:10.1021/jz101426x. ISSN 1948-7185.
- ↑ Piliarik, Marek; Sandoghdar, Vahid (2014-07-29). "Direct optical sensing of single unlabelled proteins and super-resolution imaging of their binding sites". Nature Communications 5 (1): 4495. doi:10.1038/ncomms5495. ISSN 2041-1723. PMID 25072241. Bibcode: 2014NatCo...5.4495P.
- ↑ Dahmardeh, Mahyar; Mirzaalian Dastjerdi, Houman; Mazal, Hisham; Köstler, Harald; Sandoghdar, Vahid (2023-02-27). "Self-supervised machine learning pushes the sensitivity limit in label-free detection of single proteins below 10 kDa". Nature Methods 20 (3): 442–447. doi:10.1038/s41592-023-01778-2. ISSN 1548-7105. PMID 36849549.
- ↑ Spillane, Katelyn M.; Ortega-Arroyo, Jaime; de Wit, Gabrielle; Eggeling, Christian; Ewers, Helge; Wallace, Mark I.; Kukura, Philipp (2014-08-27). "High-Speed Single-Particle Tracking of GM1 in Model Membranes Reveals Anomalous Diffusion due to Interleaflet Coupling and Molecular Pinning". Nano Letters 14 (9): 5390–5397. doi:10.1021/nl502536u. ISSN 1530-6984. PMID 25133992. Bibcode: 2014NanoL..14.5390S.
- ↑ Lavars, Nick (June 24, 2021). "Real-time view of lithium ions in motion opens door to next-gen batteries" (in en-US). https://newatlas.com/energy/real-time-view-lithium-ions-next-gen-batteries/.
 |