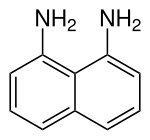
Chemistry:1,8-Diaminonaphthalene
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
Naphthalene-1,8-diamine | |
| Other names
Deltamin, 1,8-Naphthalenediamine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H10N2 | |
| Molar mass | 158.1998 |
| Related compounds | |
Related Aromatic amines
|
1-Naphthylamine 1,8-bis(dimethylamino)naphthalene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
1,8-Diaminonaphthalene is an organic compound with the formula C10H6(NH2)2. It is one of several isomeric naphthalenediamines. It is a colorless solid that darkens in air due to oxidation. It is a precursor to commercial pigments.[1]
Synthesis and reactions
It is prepared by reduction of 1,8-dinitronaphthalene, which in turn is obtained as a mixture of isomers by nitration of 1-nitronaphthalene.
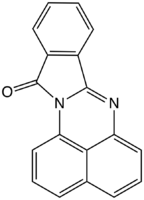
Upon treatment with phthalic anhydride derivatives, the diamine converts to phthaloperinones.[2] The derivative from phthalic anhydride itself, Solvent Orange 60, is a useful orange pigment. It is a precursor to 1,8-bis(dimethylamino)naphthalene. This compound used to produce perimidines by various aldehydes.[3]
See also
References
- ↑ Booth, Gerald (2005). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH..
- ↑ Mamada, Masashi; PéRez-BolíVar, César; Anzenbacher, Pavel (2011). "Green Synthesis of Polycyclic Benzimidazole Derivatives and Organic Semiconductors". Organic Letters 13 (18): 4882–4885. doi:10.1021/ol201973w. PMID 21863817. https://scholarworks.bgsu.edu/chem_pub/145.
- ↑ F.K. Behbahani, F.M.Golchin (2017). "A new catalyst for the synthesis of 2-substituted perimidines catalysed by FePO4". Journal of Taibah University for Science 11: 85–89. doi:10.1016/j.jtusci.2015.10.004.
 |