Chemistry:Cadet's fuming liquid
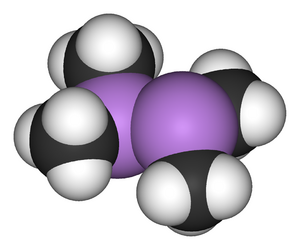
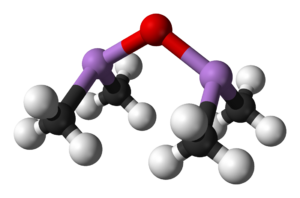
Cadet's fuming liquid was a red-brown oily liquid prepared in 1760 by the French chemist Louis Claude Cadet de Gassicourt (1731-1799) by the reaction of potassium acetate with arsenic trioxide.[1] It consisted mostly of dicacodyl (((CH3)2As)2) and cacodyl oxide (((CH3)2As)2O).
The global reaction (mass balance) corresponding to the oxide formation is the following:
- 4 CH
3COOK + As
2O
3 → ((CH
3)
2As)
2O + 2 K
2CO
3 + 2 CO
2
These were the first organometallic substances prepared; as such, Cadet has been regarded as the father of organometallic chemistry.[2]
This liquid develops white fumes when exposed to air, resulting in a pale flame producing carbon dioxide, water, and arsenic trioxide. It has a nauseating and very disagreeable garlic-like odor.
Around 1840, Robert Bunsen did much work on characterizing the compounds in the liquid and its derivatives. His research was important in the development of radical theory.
References
- ↑ Seyferth, D. (2001). "Cadet's Fuming Arsenical Liquid and the Cacodyl Compounds of Bunsen". Organometallics 20 (8): 1488–1498. doi:10.1021/om0101947.
- ↑ Jaouen, G. (2006). Bioorganometallics: Biomolecules, Labeling, Medicine. Wiley. ISBN 3-527-30990-X.
 |