Chemistry:Bismabenzene
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
Bismine | |
| Other names
Bismin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H5Bi | |
| Molar mass | 274.075 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
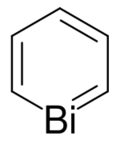
Bismabenzene (C
5H
5Bi) is the parent representative of a group of organobismuth compounds that are related to benzene with a carbon atom replaced by a bismuth atom. Bismabenzene itself has been synthesised but not isolated because it is too reactive, tending to instead dimerize in a Diels-Alder addition.[1][2][3]

Bond lengths and angles of benzene, pyridine, phosphorine, arsabenzene, stibabenzene, and bismabenzene[clarification needed]
An unstable derivative with 4-alkyl substituents was reported in 1982.[4] A stable derivative, with two ortho tri(isopropyl)silyl substituents, was synthesized from aluminacyclohexadiene, bismuth trichloride, and DBU in 2016.[1][5]
References
- ↑ 1.0 1.1 Fernando Gomollon-Bel (September 29, 2016). "Chemists create stable bismuth benzene derivative". Chemistry World. https://www.chemistryworld.com/news/chemists-create-stable-bismuth-benzene-derivative/1017447.article.
- ↑ Ashe, Arthur J.; Gordon, Michael D. (1972). "Bismabenzene. Reaction of Group V heteroaromatic compounds with hexafluorobutyne". Journal of the American Chemical Society 94 (21): 7596–7597. doi:10.1021/ja00776a063.
- ↑ Ashe, A. J. (1978). "The Group 5 Heterobenzenes". Accounts of Chemical Research 11 (4): 156. doi:10.1021/ar50124a005. "Bismabenzene is so reactive that is exists in the form of a Diels-Alder dimer (10f) at low temperature (<-10 °C).".
- ↑ Ashe, Arthur J.; Diephouse, Timothy R.; El-Sheikh, Maher Y. (1982). "Stabilization of stibabenzene and bismabenzene by 4-alkyl substituents". Journal of the American Chemical Society 104 (21): 5693–5699. doi:10.1021/ja00385a024.
- ↑ Ishii, Takuya; Suzuki, Katsunori; Nakamura, Taichi; Yamashita, Makoto (2016). "An Isolable Bismabenzene: Synthesis, Structure, and Reactivity". Journal of the American Chemical Society 138 (39): 12787–12790. doi:10.1021/jacs.6b08714. PMID 27654463. https://figshare.com/articles/journal_contribution/3859389.
 |

