Chemistry:Tipelukast
From HandWiki
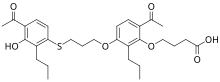
| |
| Names | |
|---|---|
| IUPAC name
4-[6-acetyl-3-[3-(4-acetyl-3-hydroxy-2-propylphenyl)sulfanylpropoxy]-2-propylphenoxy]butanoic acid
| |
| Other names
KCA 757; MN-001
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C29H38O7S | |
| Molar mass | 530.68 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tipelukast (KCA 757 or MN-001) is a sulfidopeptide leukotriene receptor antagonist with suspected anti-inflammatory properties. It is developed by MediciniNova.[1][2][3][4]
References
- ↑ "MN-001 - MediciNova, Inc.". https://medicinova.com/clinical-development/core/mn-001-nash/.
- ↑ Rajasekaran, Mahadevan; Locke, Kenneth W.; Parsons, C. Lowell (August 2006). "MN‐001, a novel oral anti‐inflammatory agent, suppresses bladder hyperactivity in a rat model". BJU International 98 (2): 430–434. doi:10.1111/j.1464-410X.2006.06274.x. PMID 16879690.
- ↑ Matsuda, Kazuko; Iwaki, Yuichi (December 2014). "MN-001 (tipelukast), a novel, orally bioavailable drug, reduces fibrosis and inflammation and down-regulates TIMP-1, collagen Type 1 and LOXL2 mRNA overexpression in an advanced NASH (nonalcoholic steatohepatitis) model: LB-28". Hepatology 60 (6): 1283A. ISSN 0270-9139. https://journals.lww.com/hep/citation/2014/12000/mn_001__tipelukast_,_a_novel,_orally_bioavailable.76.aspx.
- ↑ Bascom, R.; Hitz, K.; Dimmock, A.E.F.; Makhay, M.; Dojillo, J.; Matsuda, K. (May 2020). "Description of Protocol to Evaluate MN-001'S (tipelukast) Efficacy, Safety and Tolerability in Subjects with Idiopathic Pulmonary Fibrosis". A37. Ild Therapy I. pp. A1493. doi:10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A1493.
 |

