Chemistry:Mavoglurant
| |
| Names | |
|---|---|
| Preferred IUPAC name
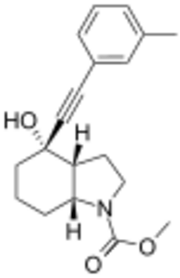
Methyl (3aR,4S,7aR)-4-hydroxy-4-[(3-methylphenyl)ethynyl]octahydro-1H-indole-1-carboxylate | |
| Other names
AFQ056
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C19H23NO3 | |
| Molar mass | 313.397 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mavoglurant (developmental code name AFQ-056) is an experimental drug candidate for the treatment of fragile X syndrome and other conditions which has been discontinued.[1][2] It exerts its effect as an antagonist of the metabotropic glutamate receptor 5 (mGluR5).[3][4][5]
Mavoglurant was under development by Novartis and reached phase II and phase III clinical trials.[2][6] Phase IIb/III dose finding and evaluation trials for fragile X-syndrome were discontinued by the end of 2014.[7] Otherwise, it would have been the first drug to treat the underlying disorder instead of the symptoms of fragile X syndrome.[8] Mavoglurant was also in phase II clinical trials for Levodopa-induced dyskinesia.[9][10] In 2007, Norvartis had conducted a clinical study to assess its ability of reducing cigarette smoking, but no results had been published up till now.[11] Novartis was conducting a clinical trial with this drug on obsessive–compulsive disorder.[12]
Novartis discontinued development of mavoglurant for fragile X syndrome in April 2014 following disappointing trial results.[7] Development was discontinued for other indications by 2017.[1]
Recently, Stalicla, a biotech company applying artificial intelligence to identify subgroups of high-responder patients, acquired worldwide rights from Novartis to progress the drug for substance-use and neurodevelopmental disorders.[13] [14][15]
See also
References
- ↑ 1.0 1.1 "Mavoglurant". AdisInsight. Springer Nature Switzerland AG. http://adisinsight.springer.com/drugs/800022397.
- ↑ 2.0 2.1 "Mavoglurant". Drugs of the Future 37 (1): 7–12. 2012. doi:10.1358/dof.2012.037.01.1772147.
- ↑ "AFQ056, a new mGluR5 antagonist for treatment of fragile X syndrome". Neurobiology of Disease 42 (3): 311–317. June 2011. doi:10.1016/j.nbd.2011.01.022. PMID 21316452.
- ↑ "Detailed In Vitro Pharmacological Characterization of Clinically Tested Negative Allosteric Modulators of the Metabotropic Glutamate Receptor 5". Molecular Pharmacology 98 (1): 49–60. July 2020. doi:10.1124/mol.119.119032. PMID 32358164.
- ↑ "Clinical investigations of compounds targeting metabotropic glutamate receptors". Pharmacology, Biochemistry, and Behavior 219: 173446. September 2022. doi:10.1016/j.pbb.2022.173446. PMID 35987339.
- ↑ "Epigenetic modification of the FMR1 gene in fragile X syndrome is associated with differential response to the mGluR5 antagonist AFQ056". Science Translational Medicine 3 (64): 64ra1. January 2011. doi:10.1126/scitranslmed.3001708. PMID 21209411.
- ↑ 7.0 7.1 "Novartis Discontinues Development of mavoglurant (AFQ056) for Fragile X Syndrome". Fragile X Research. FRAXA Research Foundation. 2014-04-24. http://www.fraxa.org/novartis-discontinues-development-mavoglurant-afq056-fragile-x-syndrome/.
- ↑ "AFQ056 drug improves symptoms in Fragile X patients: Study". news-medical.net. January 9, 2011. http://www.news-medical.net/news/20110109/AFQ056-drug-improves-symptoms-in-Fragile-X-patients-Study.aspx.
- ↑ "Mavoglurant (AFQ056) in combination with increased levodopa dosages in Parkinson's disease patients". The International Journal of Neuroscience 126 (1): 20–24. Sep 2013. doi:10.3109/00207454.2013.841685. PMID 24007304.
- ↑ "AFQ056 & Parkinson Search". ClincalTrials.gov. U.S. National Library of Medicine. http://clinicaltrials.gov/ct2/results?term=AFQ056+parkinson.
- ↑ Clinical trial number NCT00414752 for "Effects of AFQ056 and Nicotine in Reducing Cigarette Smoking" at ClinicalTrials.gov
- ↑ Clinical trial number NCT01813019 for "Study to Evaluate the Effect of AFQ056 in Obsessive Compulsive Disorder (OCD)" at ClinicalTrials.gov
- ↑ "Novartis offloads neurodevelopmental disorder asset to small Swiss biotech". Endpoints News. 2023-01-09. https://endpts.com/novartis-offloads-neurodevelopmental-disorder-asset-to-small-swiss-biotech/.
- ↑ "Stalicla Inks Mavoglurant Deal With Novartis". Inside Precison Medicine; Genetic Engineering & Biotechnology News.. Mary Ann Liebert. 2023-01-12. https://www.insideprecisionmedicine.com/news-and-features/stalicla-inks-mavoglurant-deal-with-novartis/.
- ↑ "STALICLA attracts US partner for Phase 3 development of anti-cocaine drug". Startupticker Foundation. 2023-03-13. https://www.startupticker.ch/en/news/stalicla-attracts-us-partner-for-phase-3-development-of-anti-cocaine-drug.
 |

