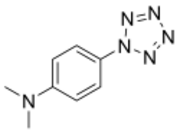
Chemistry:4-Dimethylaminophenylpentazole
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
N,N-Dimethyl-4-(1H-pentazol-1-yl)aniline | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C8H10N6 | |
| Molar mass | 190.205 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
4-Dimethylaminophenylpentazole is an unstable, explosive compound that contains the rare pentazole ring, which is composed of five nitrogen atoms. The electron donating effect of the 4-dimethylamino substituent on the phenyl ring makes this compound one of the more stable of the phenylpentazoles. At room temperature, its chemical half-life is only a few hours, although storage is possible at cryogenic temperatures. The compound was first prepared in 1956[1][2][3] along with other substituted phenylpentazoles. Studies have been conducted on various other derivatives, though necessarily limited by the instability of these compounds.[4][5][6][7][8] Some more highly substituted derivatives, such as 2,6-dihydroxy-4-dimethylaminophenylpentazole, are slightly more stable but conversely, more difficult to make.[9][10] Current research has focused on forming transition metal complexes of these pentazole derivatives, as the pentazole ring should be stabilised by bonding to the metal centre.[11][12][13]
References
- ↑ Huisgen R, I. Ugi. Zur Losung eines klassichen Problems der organischen Stickstoff-Chemie. Angewandte Chemie. 1956; 68:705-706.
- ↑ Ugi I, R. Huisgen. Pentazole II. Die Zerfallsgeschwindigkeit der Arylpentazole. Chemische Berichte. 1958; 91:531-537.
- ↑ Ugi I, Perlinger H, Perlinger L. Pentazole III. Kristallisierte Aryl-pentazole. Chemische Berichte 1958; 98:2324-2329,
- ↑ John D. Wallis and Jack D. Dunitz. An all-nitrogen aromatic ring system: structural study of 4-dimethyl-aminophenylpentazole. Journal of the Chemical Society. Chemical Communications. 1983: 910-911.
- ↑ Butler, R. N.; Collier, S.; Fleming, A. F. M. (1996). "Pentazoles: proton and carbon-13 NMR spectra of some 1-arylpentazoles: kinetics and mechanism of degradation of the arylpentazole system". Journal of the Chemical Society, Perkin Transactions 2 (5): 801. doi:10.1039/P29960000801.
- ↑ Butler, R. N.; Fox, A.; Collier, S.; Burke, L. A. (1998). "Pentazole chemistry: the mechanism of the reaction of aryldiazonium chlorides with azide ion at −80 °C: concerted versus stepwise formation of arylpentazoles, detection of a pentazene intermediate, a combined 1H and 15N NMR experimental and ab initio theoretical study". Journal of the Chemical Society, Perkin Transactions 2 (10): 2243–2248. doi:10.1039/A804040K.
- ↑ "Arylpentazoles revisited: experimental and theoretical studies of 4-hydroxyphenylpentazole and 4-oxophenylpentazole anion". The Journal of Organic Chemistry 67 (4): 1354–8. February 2002. doi:10.1021/jo0110754. PMID 11846686.
- ↑ Carlqvist, P.; Östmark, H.; Brinck, T. (2004). "The Stability of Arylpentazoles". The Journal of Physical Chemistry A 108 (36): 7463. doi:10.1021/jp0484480. Bibcode: 2004JPCA..108.7463C.
- ↑ Efforts to synthesize the pentazolate anion
- ↑ David Adam. The synthesis and characterisation of halogen and nitro phenyl azide derivatives as highly energetic materials. PhD dissertation, Ludwig-Maximilans-Universität München, 2001 [1]
- ↑ "Structure, energetics, and bonding of first row transition metal pentazolato complexes: a DFT study". Inorganic Chemistry 43 (4): 1273–86. February 2004. doi:10.1021/ic035112g. PMID 14966962.
- ↑ Burke, L. A.; Fazen, P. J. (2004). "Electronic Supplementary Information for Chemical Communications". Chemical Communications (9): 1082–3. doi:10.1039/B315812H. PMID 15116195.
- ↑ Burke, L. A.; Fazen, P. J. (2009). "Correlation analysis of the interconversion and nitrogen loss reactions of aryl pentazenes and pentazoles derived from aryl diazonium and azide ions". International Journal of Quantum Chemistry 109 (15): 3613. doi:10.1002/qua.22408. Bibcode: 2009IJQC..109.3613B.
 |

