Chemistry:Tetrabutylammonium chloride
From HandWiki
Short description: Quaternary ammonium salt of chloride
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| 3571227 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| 10839 | |
PubChem CID
|
|
| |
| |
| Properties | |
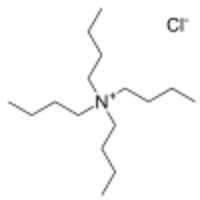
| [(CH 3CH 2CH 2CH 2) 4N]Cl | |
| Molar mass | 277.92 g·mol−1 |
| Appearance | white solid |
| Density | 1.018 g/cm3 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Tetrabutylammonium chloride is the organic compound with the formula [(CH
3CH
2CH
2CH
2)
4N]+
Cl−
, often abbreviated as [Bu
4N]Cl, where Bu stands for n-butyl. A white water-soluble solid, it is a quaternary ammonium salt of chloride. It is a precursor to other tetrabutylammonium salts.[1][2] Often tetrabutylammonium bromide is preferred as a source of tetrabutylammonium because it is less hygroscopic than the chloride.[3]
References
- ↑ Barder, T. J.; Walton, R. A. (1985). "Tetrabutylammonium Octachlorodirhenate(III)". Inorganic Syntheses. 23. 116–118. doi:10.1002/9780470132548.ch22. ISBN 9780470132548.
- ↑ Dilworth, J. R.; Hussain, W.; Hutson, A. J.; Jones, C. J.; McQuillan, F. S. (1997). "Tetrahalo Oxorhenate Anions". Inorganic Syntheses. pp. 257–262. doi:10.1002/9780470132623.ch42. ISBN 9780470132623.
- ↑ Klemperer, Walter G. (1990). "Tetrabutylammonium Isopolyoxometalates". Inorganic Syntheses. 27. pp. 74–85. doi:10.1002/9780470132586.ch15. ISBN 9780470132586.
 |

