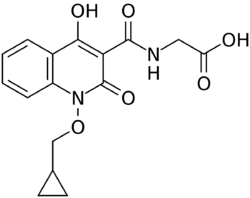
Chemistry:Desidustat
 | |
| Clinical data | |
|---|---|
| Other names | ZYAN1 |
| Identifiers | |
| |
| CAS Number | |
| UNII | |
| Chemical and physical data | |
| Formula | C16H16N2O6 |
| Molar mass | 332.312 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Desidustat (INN, also known as ZYAN1) is a drug for the treatment of anemia of chronic kidney disease. This drug with the brand name Oxemia is discovered and developed by Zydus Life Sciences.[1] Desidustat reduces the requirement of recombinant erythropoietin requirement in anemia, and decreases EPO-resistance, by reducing IL-6, IL-1β, and anti-EPO antibodies.[2] The subject expert committee of CDSCO has recommended the grant of permission for manufacturing and marketing of Desidustat 25 mg and 50 mg tablets in India,based on some conditions related to package insert, phase 4 protocols, prescription details, and GCP.[3] Clinical trials on desidustat have been done in India and Australia.[4] In a Phase 2, randomized, double-blind, 6-week, placebo-controlled, dose-ranging, safety and efficacy study, a mean hemoglobin increase of 1.57, 2.22, and 2.92 g/dL in desidustat 100, 150, and 200 mg arms, respectively, was observed.[5] The Phase 3 clinical trials were conducted in chronic kidney disease patients which were not on dialysis [6] as well as on dialysis.[7] Desidustat is developed for the treatment of anemia as an oral tablet, where currently injections of erythropoietin and its analogues are drugs of choice. Desidustat is a HIF prolyl-hydroxylase inhibitor. In preclinical studies, effects of desidustat was assessed in normal and nephrectomized rats, and in chemotherapy-induced anemia. Desidustat demonstrated hematinic potential by combined effects on endogenous erythropoietin release and efficient iron utilization.[8][9] Desidustat can also be useful in treatment of anemia of inflammation since it causes efficient erythropoiesis and hepcidin downregulation.[10] In January 2020, Zydus entered into licensing agreement with China Medical System (CMS) Holdings for development and commercialization of desidustat in Greater China. Under the license agreement, CMS will pay Zydus an initial upfront payment, regulatory milestones, sales milestones and royalties on net sales of the product. CMS will be responsible for development, registration and commercialization of desidustat in Greater China.[11] It has been observed that desidustat protects against acute and chronic kidney injury by reducing inflammatory cytokines like IL-6 and oxidative stress. [12] A clinical trial to evaluate the efficacy and safety of desidustat tablet for the management of COVID-19 patients is ongoing in Mexico, wherein desidustat has shown to prevent acute respiratory distress syndrome (ARDS) by inhibiting IL-6.[13] Zydus has also received approval from the US FDA to initiate clinical trials of desidustat in chemotherapy Induced anemia (CIA).[14]
References
- ↑ "Zydus receives DCGI approval for new drug Oxemia; what you need to know". https://www.businesstoday.in/industry/pharma/story/zydus-receives-dcgi-approval-for-new-drug-oxemia-what-you-need-to-know-324966-2022-03-07.
- ↑ Joharapurkar A.A., Patel V.J.,Kshirsagar S.G., Patel M.S., Savsani H.H., Kajavadara C., Valani D., Prolyl hydroxylase inhibitor desidustat improves anemia in erythropoietin hyporesponsive state, Current Research in Pharmacology and Drug Discovery, Available online 30 April 2022, 100102, https://doi.org/10.1016/j.crphar.2022.100102
- ↑ CDSCO, SEC Committee. "SEC meeting to examine IND proposals, dated 29.12.2021". CDSCO. https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/common_download.jsp?num_id_pk=MTU4NQ==.
- ↑ "Phase I Clinical Study of ZYAN1, A Novel Prolyl-Hydroxylase (PHD) Inhibitor to Evaluate the Safety, Tolerability, and Pharmacokinetics Following Oral Administration in Healthy Volunteers". Clinical Pharmacokinetics 57 (1): 87–102. January 2018. doi:10.1007/s40262-017-0551-3. PMID 28508936.
- ↑ Parmar DV, Kansagra KA, Patel JC, Joshi SN, Sharma NS, Shelat AD, Patel NB, Nakrani VB, Shaikh FA, Patel HV; on behalf of the ZYAN1 Trial Investigators. Outcomes of Desidustat Treatment in People with Anemia and Chronic Kidney Disease: A Phase 2 Study. Am J Nephrol. 2019 May 21;49(6):470-478. doi: 10.1159/000500232.
- ↑ Agrawal D, Varade D, Shah H, Nazar A, Krishnan J, Shukla V, Ramakrishna C, Bandara Galahitiyawa MC, Mavani SB, Rajanna S, Jikki P, De Silva S, Ruhela V, Koradia P, Kansagra K, Kanani P, Sharma N, Zala K, Parmar D; Study Investigator Group. Desidustat in Anemia due to Non-Dialysis-Dependent Chronic Kidney Disease: A Phase 3 Study (DREAM-ND). Am J Nephrol. 2022 Apr 22:1-9. doi: 10.1159/000523961. Epub ahead of print. PMID 35462372.
- ↑ Gang S, Khetan P, Varade D, Chinta VR, Mavani S, Gupta U, Reddy SVK, Rajanna S, Jeloka T, Ruhela V, Kansagra K, Kanani P, Bhatt J, Zala K; Study Investigator Group. Desidustat in Anemia due to Dialysis-Dependent Chronic Kidney Disease: A Phase 3 Study (DREAM-D). Am J Nephrol. 2022 Apr 22:1-9. doi: 10.1159/000523949. Epub ahead of print. PMID 35462369.
- ↑ "Pharmacological Characterization of ZYAN1, a Novel Prolyl Hydroxylase Inhibitor for the Treatment of Anemia". Drug Research 66 (2): 107–12. February 2016. doi:10.1055/s-0035-1554630. PMID 26367279.
- ↑ "Prolyl Hydroxylase Inhibitors: A Breakthrough in the Therapy of Anemia Associated with Chronic Diseases". Journal of Medicinal Chemistry 61 (16): 6964–6982. August 2018. doi:10.1021/acs.jmedchem.7b01686. PMID 29712435.
- ↑ "Pharmacological inhibition of prolyl hydroxylase protects against inflammation-induced anemia via efficient erythropoiesis and hepcidin downregulation". European Journal of Pharmacology 843: 113–120. January 2019. doi:10.1016/j.ejphar.2018.11.023. PMID 30458168.
- ↑ "Zydus enters into licensing agreement with China Medical System Holdings". Business Standard India. 20 January 2020. https://www.business-standard.com/article/news-cm/zydus-enters-into-licensing-agreement-with-china-medical-system-holdings-120012000752_1.html.
- ↑ "Prolyl hydroxylase inhibitor desidustat protects against acute and chronic kidney injury by reducing inflammatory cytokines and oxidative stress". Drug Development Research 82 (6): 852–860. September 2021. doi:10.1002/ddr.21792. PMID 33480036.
- ↑ "Zydus' trials of Desidustat shows positive results for Covid-19 management". The Hindu. https://www.thehindubusinessline.com/companies/zydus-trials-of-desidustat-shows-positive-results-for-covid-19-management/article33654437.ece.
- ↑ "Zydus receives approval from USFDA to initiate clinical trials of Desidustat in cancer patients receiving chemotherapy". La Merie Publishing. https://pipelinereview.com/index.php/2020072375375/Small-Molecules/Zydus-receives-approval-from-USFDA-to-initiate-clinical-trials-of-Desidustat-in-cancer-patients-receiving-chemotherapy.html.
 |

