Chemistry:Stevensine
| |
| Names | |
|---|---|
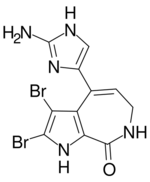
| Preferred IUPAC name
4-(2-Amino-1H-imidazol-5-yl)-2,3-dibromo-6,7-dihydropyrrolo[2,3-c]azepin-8(1H)-one | |
| Other names
Odiline
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C11H9Br2N5O | |
| Molar mass | 387.035 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Stevensine is a bromopyrrole alkaloid originally isolated from an unidentified Micronesian marine sponge, as well as a New Caledonian sponge, Pseudaxinyssa cantharella[1] and Axinella corrugata.[2][3] Total synthesis of stevensine has been achieved by Ying-zi Xu et al.,[4] and investigations into the biosynthetic origin has been explored by Paul Andrade et al.[1] Understanding methods to synthesize stevensine and other similar compounds is an important step to accomplish, as marine sponges contain numerous biologically active metabolites that have been shown to function as anything from antitumor to antibacterial agents when tested for medicinal applications.[2] Reasons for why marine sponges contain so many bio-active chemicals has been attributed to their sessile nature, and the need to produce chemical defenses to ensure survival.[5] However, since many of these compounds naturally occur in small amounts, harvesting the sponges has in the past led to near-extinction of some species.
The bioactive nature of stevensine has been explored both as to its evolutionary purpose as well as potential medicinal uses. At its natural concentrations in vivo, stevensine, as well as other secondary metabolite bromopyrroles from sponges have been shown to function as anti-feeding agents against predatory fish such as bluehead wrasse (Thalassoma bifasciatum).[3] Stevensine is present in marine sponges in concentrations of approximately 19 mg/mL, but have been shown to deter feeding in a laboratory setting in concentrations as low as 2.25 mg/mL, while deterring in the field requires as much as 12 mg/mL. In vitro tests have shown that this compound functions as an antimicrobial agent,[6] giving promise for this compound to be used as a potential drug, however it does not lower the activity of methicillin-resistant Staphylococcus aureus (MRSA), while related compounds isolated from sponges such as bromoageliferin do.[5]
References
- ↑ 1.0 1.1 Andrade, Paul; Willoughby, Robin; Pomponi, Shirley A.; Kerr, Russell G. (1999-06-25). "Biosynthetic studies of the alkaloid, stevensine, in a cell culture of the marine sponge Teichaxinella morchella". Tetrahedron Letters 40 (26): 4775–4778. doi:10.1016/S0040-4039(99)00881-3. ISSN 0040-4039.
- ↑ 2.0 2.1 Duckworth, Alan R.; Samples, Gail A.; Wright, Amy E.; Pomponi, Shirley A. (2003-12-01). "In Vitro CuIture of the Tropical Sponge Axinella corrugata (Demospongiae): Effect of Food Cell Concentration on Growth, Clearance Rate, and Biosynthesis of Stevensine". Marine Biotechnology 5 (6): 519–527. doi:10.1007/s10126-002-0111-0. ISSN 1436-2228. PMID 14564533. Bibcode: 2003MarBt...5..519D.
- ↑ 3.0 3.1 Wilson, Dean M.; Puyana, Monica; Fenical, William; Pawlik, Joseph R. (1999-12-01). "Chemical Defense of the Caribbean Reef Sponge Axinella corrugata Against Predatory Fishes". Journal of Chemical Ecology 25 (12): 2811–2823. doi:10.1023/A:1020811810223. ISSN 1573-1561.
- ↑ Xu, Ying-zi; Yakushijin, Kenichi; Horne, David A. (February 1997). "Synthesis of C11N5Marine Sponge Alkaloids: (±)-Hymenin, Stevensine, Hymenialdisine, and Debromohymenialdisine†". The Journal of Organic Chemistry 62 (3): 456–464. doi:10.1021/jo9619746. ISSN 0022-3263. PMID 11671434.
- ↑ 5.0 5.1 Melander, Roberta J.; Liu, Hong-bing; Stephens, Matthew D.; Bewley, Carole A.; Melander, Christian (2016-12-15). "Marine sponge alkaloids as a source of anti-bacterial adjuvants". Bioorganic & Medicinal Chemistry Letters 26 (24): 5863–5866. doi:10.1016/j.bmcl.2016.11.018. ISSN 0960-894X. PMID 27876320.
- ↑ Yalçın, Funda Nuray (January 2007). "Biological Activities of the Marine Sponge Axinella". Hacettepe University Journal of the Faculty of Pharmacy 27: 47–60. http://pdfs.semanticscholar.org/b14a/53cb18040b37d0624cbbfaaf3033ce167a04.pdf.
External links
- Mohammed, R.; Peng, J.; Kelly, M.; Hamann, M. T. (2006). "Cyclic heptapeptides from the Jamaican sponge Stylissa caribica". Journal of Natural Products 69 (12): 1739–1744. doi:10.1021/np060006n. PMID 17190452.
 |

