Chemistry:Polonium tetrabromide
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
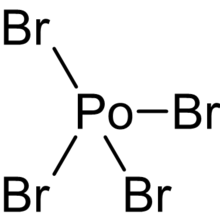
| Br4Po | |
| Molar mass | 529 g·mol−1 |
| Appearance | pale red solid[1] |
| Solubility | soluble in ethanol[2] soluble in bromine[2] |
| Structure | |
| cubic crystal system | |
| Fm3m (No. 225) | |
a = 5.6 Å
| |
| Related compounds | |
Other anions
|
polonium tetrafluoride polonium tetrachloride polonium tetraiodide |
Other cations
|
selenium tetrabromide tellurium tetrabromide |
Related compounds
|
polonium tetrabromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Polonium tetrabromide, is a bromide of polonium, with the chemical formula PoBr4.
Preparation
Polonium tetrabromide can be formed by the direct reaction of bromine and polonium at 200 °C to 250 °C.[2]
Like polonium tetraiodide, polonium tetrabromide can also be produced by the reaction of polonium dioxide and hydrogen bromide:[2]
- PoO
2 + 4 HBr → PoBr
4 + 2 H
2O
Properties
Polonium tetrabromide is a light red solid that is easily deliquescent.[1] It crystallizes in the cubic crystal system, with space group Fm3m (No. 225) and lattice parameter a = 5.6 Å.[3]
References
- ↑ 1.0 1.1 P. E. Figgins (1961), The Radiochemistry of Polonium, National Academies, pp. 13
- ↑ 2.0 2.1 2.2 2.3 M. Schmidt, W. Siebert, K. W. Bagnall (October 2013). The Chemistry of Sulphur, Selenium, Tellurium and Polonium: Pergamon Texts in Inorganic Chemistry. Elsevier. p. 960-961. ISBN 978-1483158655.
- ↑ H. J. Emeléus, A. G. Sharpe (January 1962). Advances in Inorganic Chemistry and Radiochemistry. Academic Press. p. 216. ISBN 0080578535.
 |

