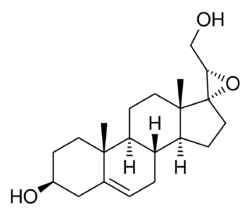
Chemistry:BNN-27
 | |
| Clinical data | |
|---|---|
| Other names | BNN27; (20R)-3β,21-Dihydroxy-17α,20-epoxypregn-5-ene; 17α,20R-Epoxypregn-5-ene-3β,21-diol |
| Identifiers | |
| |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C21H32O3 |
| Molar mass | 332.484 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
BNN-27, also known as 17α,20R-epoxypregn-5-ene-3β,21-diol, is a synthetic neurosteroid and "microneurotrophin" and analogue of the endogenous neurosteroid dehydroepiandrosterone (DHEA).[1][2][3][4] It acts as a selective, high-affinity, centrally active agonist of the TrkA and p75NTR, receptors for nerve growth factor (NGF) and other neurotrophins, as well as for DHEA and DHEA sulfate (DHEA-S).[2][3][5] BNN-27 has neuroprotective and neurogenic effects and has been suggested as a potential novel treatment for neurodegenerative diseases and brain trauma.[2][3]
In 2011, the surprising discovery was made that DHEA, as well as DHEA-S, directly bind to and activate the TrkA and p75NTR with high affinity.[5] DHEA was subsequently also found to bind to the TrkB and TrkC with high affinity, though it notably activated the TrkC but not the TrkB.[6] DHEA and DHEA-S bound to these receptors with affinities that were in the low nanomolar range (around 5 nM), although the affinities were nonetheless approximately two orders of magnitude lower relative to the highly potent polypeptide neurotrophins (0.01–0.1 nM).[5][6] In any case, DHEA and DHEA-S were identified as important endogenous neurotrophic factors.[5] These findings may explain the positive association between decreased circulating DHEA levels with age and age-related neurodegenerative diseases.[5]
Subsequently, a series of spiro derivatives of DHEA that had been synthesized and assessed in 2009 as potential neuroprotective agents was re-investigated.[1][2][3] Of these, BNN-27 was assayed and found to directly bind to and activate the TrkA and p75NTR.[2][3] In addition, it was found to cross the blood–brain barrier and to have strong neuroprotective and neurogenic effects in mouse models of neurotoxicity and neurodegeneration.[2][3] Moreover, unlike DHEA, it lacked any hormonal actions.[2][3] Also, it was found to lack the problematic hyperalgesic actions of NGF.[2][3] As such, BNN-27 has been described as an NGF mimetic and was proposed as a potential novel treatment for neurodegenerative diseases and brain trauma.[2][3]
See also
References
- ↑ 1.0 1.1 "Novel dehydroepiandrosterone derivatives with antiapoptotic, neuroprotective activity". J. Med. Chem. 52 (21): 6569–87. 2009. doi:10.1021/jm900468p. PMID 19845386.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 "Selective and differential interactions of BNN27, a novel C17-spiroepoxy steroid derivative, with TrkA receptors, regulating neuronal survival and differentiation". Neuropharmacology 111: 266–282. 2016. doi:10.1016/j.neuropharm.2016.09.007. PMID 27618740. https://zenodo.org/record/895789.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 "BNN27, a 17-Spiroepoxy Steroid Derivative, Interacts With and Activates p75 Neurotrophin Receptor, Rescuing Cerebellar Granule Neurons from Apoptosis". Front Pharmacol 7: 512. 2016. doi:10.3389/fphar.2016.00512. PMID 28082899.
- ↑ "The novel dehydroepiandrosterone (DHEA) derivative BNN27 counteracts delay-dependent and scopolamine-induced recognition memory deficits in rats". Neurobiol Learn Mem 140: 145–153. 2017. doi:10.1016/j.nlm.2017.03.004. PMID 28274826.
- ↑ 5.0 5.1 5.2 5.3 5.4 "Neurosteroid dehydroepiandrosterone interacts with nerve growth factor (NGF) receptors, preventing neuronal apoptosis". PLOS Biol. 9 (4): e1001051. 2011. doi:10.1371/journal.pbio.1001051. PMID 21541365.
- ↑ 6.0 6.1 "Dehydroepiandrosterone: an ancestral ligand of neurotrophin receptors". Endocrinology 156 (1): 16–23. 2015. doi:10.1210/en.2014-1596. PMID 25330101. https://zenodo.org/record/894291.
 |

