Chemistry:Phlorofucofuroeckol A
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
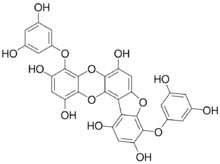
4,9-Bis(3,5-dihydroxyphenoxy)[1]benzofuro[3,2-a]oxanthrene-1,3,6,10,12-pentol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C30H18O14 | |
| Molar mass | 602.45 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Phlorofucofuroeckol A is a phlorotannin isolated from brown algae species such as Eisenia bicyclis (an edible seaweed called arame in Japan),[1] Ecklonia cava,[2] Ecklonia kurome[3] or Ecklonia stolonifera.[4]
The molecule possesses both the dibenzo-1,4-dioxin and dibenzofuran elements.[3]
References
- ↑ Eom, Sung-Hwan; Lee, Sang-Hoon; Yoon, Na-Young; Jung, Won-Kyo; Jeon, You-Jin; Kim, Se-Kwon; Lee, Myung-Suk; Kim, Young-Mog (2012). "Α-Glucosidase- and α-amylase-inhibitory activities of phlorotannins from Eisenia bicyclis". Journal of the Science of Food and Agriculture 92 (10): 2084–90. doi:10.1002/jsfa.5585. PMID 22271637.
- ↑ Ahn, MJ; Yoon, KD; Min, SY; Lee, JS; Kim, JH; Kim, TG; Kim, SH; Kim, NG et al. (2004). "Inhibition of HIV-1 reverse transcriptase and protease by phlorotannins from the brown alga Ecklonia cava". Biological & Pharmaceutical Bulletin 27 (4): 544–7. doi:10.1248/bpb.27.544. PMID 15056863.
- ↑ 3.0 3.1 Fukuyama, Y; Kodama, M; Miura, I; Kinzyo, Z; Mori, H; Nakayama, Y; Takahashi, M (1990). "Anti-plasmin inhibitor. VI. Structure of phlorofucofuroeckol A, a novel phlorotannin with both dibenzo-1,4-dioxin and dibenzofuran elements, from Ecklonia kurome Okamura". Chemical & Pharmaceutical Bulletin 38 (1): 133–5. doi:10.1248/cpb.38.133. PMID 2337936.
- ↑ Kim, AR; Lee, MS; Shin, TS; Hua, H; Jang, BC; Choi, JS; Byun, DS; Utsuki, T et al. (2011). "Phlorofucofuroeckol a inhibits the LPS-stimulated iNOS and COX-2 expressions in macrophages via inhibition of NF-κB, Akt, and p38 MAPK". Toxicology in Vitro 25 (8): 1789–95. doi:10.1016/j.tiv.2011.09.012. PMID 21963823.
 |

