Chemistry:Echinomycin

| |
| Names | |
|---|---|
| Other names
Quinomycin A; Levomycin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C51H64N12O12S2 | |
| Molar mass | 1101.26 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Echinomycin is a peptide antibiotic. It is a dimer of two peptides creating a cyclic structure. It contains a bicyclic aromatic chromophore that is attached to the dimerized cyclic peptide core and a thioacetal bridge. It intercalates into DNA at two specific sites, thereby blocking the binding of hypoxia inducible factor 1 alpha (HIF1alpha).[1]
Biosynthesis
Echinomycin is a bis-intercalator peptide and is biosynthesized by a unique nonribosomal peptide synthetase (NRPS).[2][3] Echinomycin is isolated from various bacteria such as Streptomyces lasalienis. It belongs to a family of quinoxaline antibiotics. There is great interest in this group of compounds because they have very potent antibacterial, anticancer, and antiviral activities.[4]
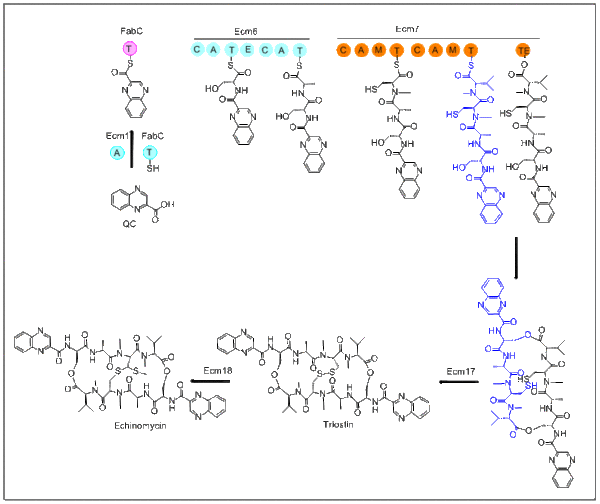
The biosynthesis of echinomycin starts with molecule QC. L-tryptophan is the precursor for QC and its biosynthesis parallels the first stage of nikkomycin biosynthesis.[citation needed]
After QC is biosynthesized, the adenylation domain-containing Ecm1 activates and transfers QC to FabC using the fatty acid biosynthesis acyl carrier protein (ACP). The first module, Ecm6 accepts the QC-SFabC as the starter unit. Emc7 contains a terminal thioesterase domain which allows the peptide to dimerize and then release. This cyclized product then goes on to Ecm17, an oxidoreductase, creating a disulfide bond. The last step in this biosynthesis transforms the disulfide bond into a thioacetal bridge. This transformation takes place within Ecm18, a S-adenosyl-L-methionine (SAM)-dependent methyltransferase.[4] The mechanism is proposed to proceed through two steps. Initially Emc18 transfers the activated methyl group from SAM to one of the sulfur atoms in the disulfide bond. Secondly deprotonation of the alpha proton to the tertiary sulfonium cation promotes the rearrangement for the formation of the thioacetal bond.[citation needed]
References
- ↑ Kong, D.; Park, E. J.; Stephen, A.G.; Mellilo, G. (1 October 2005). "Echinomycin, a Small-Molecule Inhibitor of Hypoxia-Inducible Factor-1 DNA-Binding Activity". Cancer Research 65 (19): 9047–9055. doi:10.1158/0008-5472.CAN-05-1235. PMID 16204079. https://aacrjournals.org/cancerres/article/65/19/9047/518486/Echinomycin-a-Small-Molecule-Inhibitor-of-Hypoxia. Retrieved 15 June 2022.
- ↑ Watanabe, K; Oguri, H; Oikawa, H (April 2009). "Diversification of echinomycin molecular structure by way of chemoenzymatic synthesis and heterologous expression of the engineered echinomycin biosynthetic pathway.". Current Opinion in Chemical Biology 13 (2): 189–96. doi:10.1016/j.cbpa.2009.02.012. PMID 19278894.
- ↑ "Echinomycin biosynthesis". Current Opinion in Chemical Biology 17 (4): 537–45. 2013. doi:10.1016/j.cbpa.2013.06.022. PMID 23856054.
- ↑ 4.0 4.1 Watanabe, K (August 2006). "Total biosynthesis of antitumor nonribosomal peptides in Escherichia coli.". Nature Chemical Biology 2 (8): 423–8. doi:10.1038/nchembio803. PMID 16799553.
 |