Chemistry:Stannabenzene
|
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Stannine | |||
| Other names
Stannin
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| C5H6Sn | |||
| Molar mass | 184.813 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Stannabenzene (C5H6Sn) is the parent representative of a group of organotin compounds that are related to benzene with a carbon atom replaced by a tin atom. Stannabenzene itself has been studied by computational chemistry,[1] but has not been isolated.
Stable derivatives of stannabenzene
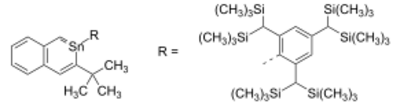
Stable derivatives of stannabenzene have been isolated. The 2-stannanaphthalene depicted below is stable in an inert atmosphere at temperatures below 140 °C.[2] The tin to carbon bond in this compound is shielded from potential reactants by two very bulky groups, one tert-butyl group and the even larger 2,4,6-tris[bis(trimethylsilyl)methyl]phenyl or Tbt group. The two Sn-C bonds have bond lengths of 202.9 and 208.1 pm which are shorter than those for Sn-C single bonds (214 pm) and comparable to that of known Sn=C double bonds (201.6 pm). The C-C bonds show little variation with bond lengths between 135.6 and 144.3 pm signaling that this compound is aromatic.
Tbt-substituted 9-stannaphenanthrene was reported in 2005.[3] At room temperature it forms the [4+2] cycloadduct.
Tbt-substituted stannabenzene was reported in 2010.[4] At room-temperature it quantitatively forms the DA dimer.
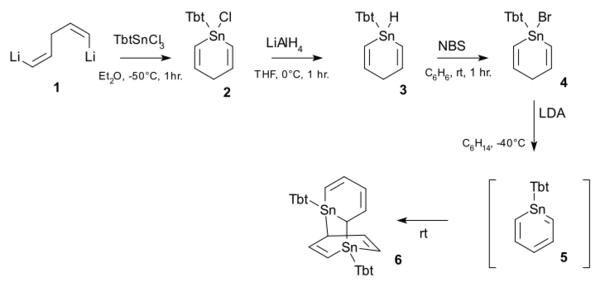 Tbt-substituted stannabenzene synthesis. Reagents lithium aluminium hydride (step 2), NBS (step 3), LDA (step 4)
Tbt-substituted stannabenzene synthesis. Reagents lithium aluminium hydride (step 2), NBS (step 3), LDA (step 4)
See also
- 6-membered aromatic rings with one carbon replaced by another group: borabenzene, silabenzene, germabenzene, stannabenzene, pyridine, phosphorine, arsabenzene, bismabenzene, pyrylium, thiopyrylium, selenopyrylium, telluropyrylium
References
- ↑ Ebrahimi, Arash Afshar; Ghiasi, Reza; Foroutan-Nejad, Cina (2010). "Topological characteristics of the ring critical points and the aromaticity of groups IIIA to VIA hetero-benzenes". Journal of Molecular Structure: THEOCHEM 941 (1–3): 47–52. doi:10.1016/j.theochem.2009.10.038.
- ↑ Mizuhata, Yoshiyuki; Sasamori, Takahiro; Takeda, Nobuhiro; Tokitoh, Norihiro (2006). "A Stable Neutral Stannaaromatic Compound: Synthesis, Structure and Complexation of a Kinetically Stabilized 2-Stannanaphthalene". Journal of the American Chemical Society 128 (4): 1050–1. doi:10.1021/ja057531d. PMID 16433501.
- ↑ Generation of 9-Stannaphenanthrene and Its Reactivities Yoshiyuki Mizuhata, Nobuhiro Takeda, Takahiro Sasamori and Norihiro Tokitoh Chemistry Letters Volume 34 Number 8 Year 2005 Page 1088 doi:10.1246/cl.2005.1088
- ↑ Generation of Stannabenzenes and Their Properties Yoshiyuki Mizuhata, Naoya Noda, and Norihiro Tokitoh Organometallics, 2010, 29 (21), pp 4781–4784 doi: 10.1021/om100382n
 |