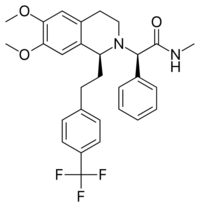
Chemistry:Almorexant
 | |
| Clinical data | |
|---|---|
| Other names | ACT-078573 |
| Routes of administration | By mouth |
| Drug class | Orexin antagonist |
| ATC code |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 13–19 Hours[1][2] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C29H31F3N2O3 |
| Molar mass | 512.573 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Almorexant, also known by its development code ACT-078573, is an orexin antagonist, acting as a competitive antagonist of the OX1 and OX2 orexin receptors, which was being developed by the pharmaceutical companies Actelion and GSK for the treatment of insomnia.[3] Development of the drug was abandoned in January 2011 due to concerns over the hepatic safety of almorexant after transient increases in liver enzymes were observed in trials.[4][5]
Pharmacology
Pharmacodynamics
Almorexant is a competitive, dual OX1 and OX2 receptor antagonist and selectively inhibits the functional consequences of OX1 and OX2 receptor activation, such as intracellular Ca2+ mobilization. It dissociates very slowly from the orexin receptors and this may prolong its duration of action.[6]
History
Originally developed by Actelion, from 2007 almorexant was being reported as a potential blockbuster drug, as its novel mechanism of action (orexin receptor antagonism) was thought to produce better quality sleep and fewer side effects than the traditional benzodiazepines and Z-drugs which dominated the multibillion-dollar insomnia medication market.[7]
In 2008, GlaxoSmithKline bought the development and marketing rights for almorexant from Actelion for an initial payment of $147 million.[8] The deal would have been worth an estimated $3.2 billion if the drug had successfully completed clinical development and obtained FDA approval.[9] GSK and Actelion continued to develop the drug together, and completed a Phase III clinical trial in November 2009.[10]
However, in January 2011 Actelion and GSK announced they were abandoning the development of almorexant because of its side effect profile.[4][11]
In 2014 researchers from Actelion published work indicating that Almorexant had mild abuse potential but significantly less abuse potential than zolpidem.[12]
Society and culture
Names
Almorexant is the generic name of the drug and its INN. It is also known by its former developmental code name ACT-078573.
References
- ↑ "Orexin Receptor Antagonists: Historical Perspectives and Future Opportunities". Current Topics in Medicinal Chemistry 16 (29): 3438–3469. 2016. doi:10.2174/1568026616666150929111607. PMID 26416477.
- ↑ "Orexin receptor antagonism, a new sleep-promoting paradigm: an ascending single-dose study with almorexant". Clinical Pharmacology and Therapeutics 87 (5): 593–600. May 2010. doi:10.1038/clpt.2010.19. PMID 20376002.
- ↑ "Almorexant, a dual orexin receptor antagonist for the treatment of insomnia". Current Opinion in Investigational Drugs 11 (1): 101–110. January 2010. PMID 20047164.
- ↑ 4.0 4.1 "GSK and Actelion discontinue clinical development of almorexant". GSK press release. 28 January 2011. http://www.gsk.com/media/pressreleases/2011/2011_pressrelease_10019.htm.
- ↑ "Entry-into-humans study with ACT-462206, a novel dual orexin receptor antagonist, comparing its pharmacodynamics with almorexant". Journal of Clinical Pharmacology 54 (9): 979–986. September 2014. doi:10.1002/jcph.297. PMID 24691844.
- ↑ "Hypocretins (orexins): The ultimate translational neuropeptides". Journal of Internal Medicine 291 (5): 533–556. May 2022. doi:10.1111/joim.13406. PMID 35043499.
- ↑ "Sleeping Beautifully". CBS Business Network. 24 September 2007. http://findarticles.com/p/articles/mi_hb5255/is_18/ai_n29394606/.
- ↑ "Actelion Sells Glaxo Almorexant Sleep Medicine Rights". Bloomberg. 14 July 2008. https://www.bloomberg.com/apps/news?pid=newsarchive&sid=aAS6UsbXi2M8.
- ↑ "Actelion's top dollar deal leaves doubts, and little on the horizon". EP Vantage. 14 July 2008. http://www.epvantage.com/Universal/View.aspx?type=Story&id=159945&isEPVantage=yes.
- ↑ Clinical trial number NCT00608985 for "Almorexant in Adult Subjects With Chronic Primary Insomnia (RESTORA 1)" at ClinicalTrials.gov
- ↑ "Actelion and GSK Discontinue Clinical Development of Almorexant". Actelion press release. 28 January 2011. http://www.actelion.com/en/our-company/news-and-events/index.page?newsId=1483135.
- ↑ Cruz, Hans G.; Hoever, Petra; Chakraborty, Bijan; Schoedel, Kerri; Sellers, Edward M.; Dingemanse, Jasper (April 2014). "Assessment of the Abuse Liability of a Dual Orexin Receptor Antagonist: A Crossover Study of Almorexant and Zolpidem in Recreational Drug Users". CNS Drugs 28 (4): 361–372. doi:10.1007/s40263-014-0150-x.
External links
 |

