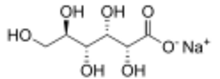
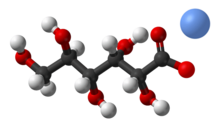
Chemistry:Sodium gluconate
| |
| |
| Names | |
|---|---|
| IUPAC name
Sodium (2R,3S,4R,5R)-2,3,4,5,6-pentahydroxyhexanoate
| |
| Other names
Sodium D-gluconate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H11NaO7 | |
| Molar mass | 218.137 g·mol−1 |
| Appearance | White powder |
| Odor | Odorless |
| 58 g/100 mL | |
| Solubility in ethanol and diethyl ether | Slightly soluble |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
10380 mg/kg (oral, rat)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium gluconate is a compound with formula NaC6H11O7.[2] It is the sodium salt of gluconic acid. Its E number is E576. This white, water-soluble powder has a wide range of applications across industries. Originally derived from gluconic acid in the 19th century, Sodium Gluconate is known for its chelating properties and is utilized as a chelating agent in various processes. It finds applications in textile, metal surface treatment, cement, and more. Moreover, its non-toxic nature and biodegradability contribute to its use in environmentally conscious practices.
Production
Sodium Gluconate can be produced through the fermentation process or chemical synthesis.[3] In the fermentation process, glucose is fermented by certain microorganisms, typically strains of Aspergillus niger or Pseudomonas. Gluconic acid is the primary product of this fermentation, and Sodium Gluconate is derived by neutralizing gluconic acid with sodium hydroxide.
Source of Gluconic Acid: The production of Sodium Gluconate commences with its precursor, gluconic acid. This organic acid is often obtained through a fermentation process. Glucose, or other sugar sources, serves as the substrate for microorganisms, typically bacteria or fungi, to produce gluconic acid.
Conversion to Sodium Gluconate: Once gluconic acid is harvested, it undergoes a transformation into Sodium Gluconate. The conversion primarily involves a chemical reaction where gluconic acid is neutralized with sodium hydroxide (NaOH). This reaction results in the formation of Sodium Gluconate, where the sodium ions (Na+) replace the hydrogen ions (H+) in gluconic acid.
Purification and Crystallization: Purification often includes filtration and chemical treatments to achieve the desired level of purity. Following purification, the solution is subjected to crystallization.
Drying and Packaging: After crystallization, the Sodium Gluconate crystals still contain residual moisture. Drying may involve processes like air drying or spray drying.
Applications
Sodium Gluconate's early uses were primarily in medicine due to its mild and non-toxic properties. Over time, its applications expanded to various industries, including food, pharmaceuticals, construction, textiles, and more, as its versatile properties and safety profile became more widely recognized.
Food Industry: Sodium Gluconate is used as a food additive for various purposes, including as a sequestrant to prevent metal ions from affecting the color, flavor, or stability of food products.
Construction: Sodium Gluconate is employed in the construction industry as a concrete admixture. It acts as a water reducer and retarder, enhancing the workability and performance of concrete.
Textiles: In textile dyeing and printing, it is utilized as a chelating agent to improve color fastness.
Metallurgy: Sodium Gluconate is employed for metal surface treatment and cleaning, particularly for steel surfaces.
Cleaning Products: It can be found in cleaning agents for glass bottles and as a chelating agent in various cleaning formulations.
Safety and Regulation
Sodium Gluconate is generally recognized as safe (GRAS) for consumption by regulatory authorities such as the U.S. Food and Drug Administration (FDA). It is considered non-toxic and safe for use in food and pharmaceuticals.
Environmental Impact
Sodium Gluconate is known for its biodegradability,[4] which means it can break down naturally in the environment. It is considered environmentally friendly.
References
- ↑ Chemistry id sis.nlm.nih.gov [|permanent dead link|dead link}}]
- ↑ "Sodium Gluconate (Chelating Agent): Cosmetic Ingredient INCI". https://cosmetics.specialchem.com/inci-ingredients/sodium-gluconate. Retrieved 18 November 2023.
- ↑ Papagianni, M. (1 January 2011). Moo-Young, Murray. ed. Comprehensive Biotechnology (Second ed.). Academic Press. pp. 109–120. https://www.sciencedirect.com/science/article/pii/B9780080885049000118. Retrieved 18 November 2023.
- ↑ Sodium Gluconate Santos.com December 2021
 |

