Chemistry:Tetrazolium chloride
| |
| |
| Names | |
|---|---|
| IUPAC name
2,3,5-Triphenyl-2H-tetrazolium chloride
| |
| Other names
TTC
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| |
| |
| Properties | |
| C19H15ClN4 | |
| Molar mass | 334.8 g/mol |
| Appearance | white crystalline powder |
| log P | −2.4 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Triphenyl tetrazolium chloride, TTC, or simply tetrazolium chloride (with the formula 2,3,5-triphenyl-2H-tetrazolium chloride) is a redox indicator commonly used in biochemical experiments especially to indicate cellular respiration. It is a white crystalline powder, soluble in water, ethanol and acetone but insoluble in ether.
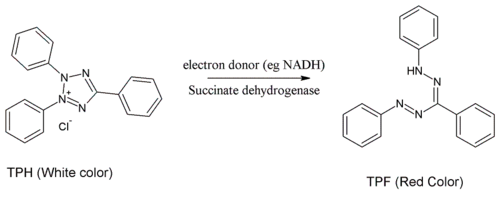
TTC assay
In the TTC assay (also known as TTC test or tetrazolium test), TTC is used to differentiate between metabolically active and inactive tissues. The white compound is enzymatically reduced to red TPF (1,3,5-triphenylformazan) in living tissues due to the activity of various dehydrogenases (enzymes important in oxidation of organic compounds and thus cellular metabolism), while it remains as white TTC in areas of necrosis since these enzymes have been either denatured or degraded.
For this reason, TTC has been employed in autopsy pathology to assist post-mortem identification of myocardial infarctions. Healthy viable heart muscle will stain deep red from the cardiac lactate dehydrogenase; while areas of potential infarctions will be more pale.
Note: TTC is somewhat heat and light unstable, so avoid these environments as much as possible.
See also
References
 |

