Chemistry:Ethenedithione
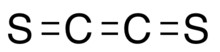
| |
| Names | |
|---|---|
| IUPAC name
Ethenedithione
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2S2 | |
| Molar mass | 88.14 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ethenedithione or ethylenedithone is an unstable chemical substance with formula S=C=C=S made from carbon and sulfur.
Ethenedithione can exist as a gas at low pressure and high temperature, but is unstable when condensed or under higher pressure.[1]
It can be stabilized as a ligand binding two cobalt atoms.[2]
Other occurrences as a ligand are in TpW(CO)2(C2S2)– and [TpW(CO)2]2Ni(C2S2)2, where Tp is trispyrazolylborate.[3]
Formation
Ethenedithione can be made by the flash vacuum pyrolysis of 2,5-Dithiacyclopentylideneketene.[1]
Also it has been made by dissociative ionization of tetrathiapentalenedione, and then neutralisation of ions produced.[4]
C2S2 is made along with carbon subsulfide and carbon monosulfide, in an electric discharge in carbon disulfide vapour.[5]
Properties
In its ground state it is a triplet state (3Σg−).[6] Ethenedithione can be trapped in a matrix of solid argon without decomposition.[1]
The infrared spectrum contains a prominent line at 1179.3 cm−1 due to asymmetric C=S stretch of the most common isotopes.[1]
Over 60K, ethenedithione polymerises.[7] Possible polymerisation products include polythiene.[8]
References
- ↑ 1.0 1.1 1.2 1.3 Wentrup, Curt; Kambouris, Peter; Evans, Richard A.; Owen, David; Macfarlane, Graham; Chuche, Josselin; Pommelet, Jean Claude; Cheikh, Abdelhamid Ben et al. (April 1991). "2,5-Dithiacyclopentylideneketene and ethenedithione, S:C:C:S, generated by flash vacuum pyrolysis". Journal of the American Chemical Society 113 (8): 3130–3135. doi:10.1021/ja00008a048.
- ↑ Seidel, Wolfram W.; Meel, Matthias J.; Hughes, Stephen R.; Hupka, Florian; Villinger, Alexander (23 December 2011). "Ethenedithione (S=C=C=S): Trapping and Isomerization in a Cobalt Complex". Angewandte Chemie International Edition 50 (52): 12617–12620. doi:10.1002/anie.201105055.
- ↑ Zhang, Zhong; Pu, Liang; Li, Qian-shu; King, R. Bruce (2014). "The facile coupling of carbon monochalcogenides to ethenedichalcogenone ligands in binuclear iron carbonyl derivatives: a theoretical study". New J. Chem. 38 (9): 4282–4289. doi:10.1039/C4NJ00740A.
- ↑ Sülzle, Detlev; Schwarz, Helmut (October 1988). "Ethylenedithione(C2S2): Generation and Characterization by Neutralization-Reionization Mass Spectrometry". Angewandte Chemie International Edition in English 27 (10): 1337–1339. doi:10.1002/anie.198813371.
- ↑ Bohn, Robert B.; Hannachi, Yacine; Andrews, Lester (July 1992). "Production and reactions of triplet CS: matrix infrared and ultraviolet spectra of C2S2". Journal of the American Chemical Society 114 (16): 6452–6459. doi:10.1021/ja00042a024.
- ↑ Ma, Ngai Ling; Wong, Ming Wah (1998-12-31). "Ethenedithione (S=C=C=S): Does It Obey Hund's Rule?" (in en). Angewandte Chemie International Edition 37 (24): 3402–3404. doi:10.1002/(SICI)1521-3773(19981231)37:24<3402::AID-ANIE3402>3.0.CO;2-S. ISSN 1433-7851.
- ↑ Yranzo, Gloria I.; Elguero, José; Flammang, Robert; Wentrup, Curt (June 2001). "Formation of Cumulenes, Triple-Bonded, and Related Compounds by Flash Vacuum Thermolysis of Five-Membered Heterocycles". European Journal of Organic Chemistry 2001 (12): 2209–2220. doi:10.1002/1099-0690(200106)2001:12<2209::AID-EJOC2209>3.0.CO;2-X.
- ↑ Zmolek, Peter B.; Sohn, Honglae; Gantzel, Peter K.; Trogler, William C. (1 February 2001). "Photopolymerization of Liquid Carbon Disulfide Produces Nanoscale Polythiene Films". Journal of the American Chemical Society 123 (6): 1199–1207. doi:10.1021/ja003200j.
 |

