Chemistry:3,3-Dimethyl-1-butanol
| |
| Names | |
|---|---|
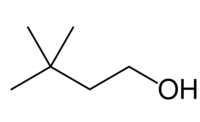
| Preferred IUPAC name
3,3-Dimethylbutan-1-ol | |
| Other names
3,3-Dimethyl-1-butanol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H14O | |
| Molar mass | 102.177 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
3,3-Dimethyl-1-butanol (DMB) is a structural analog of choline.[1]
Effects
DMB inhibits microbial trimethylamine (TMA) formation in mice and in human feces, thereby reducing plasma trimethylamine N-oxide (TMAO) levels after choline or carnitine supplementation.[1] It consequently inhibited choline-enhanced endogenous macrophage foam cell formation and atherosclerotic lesion development in mice without alterations in circulating cholesterol levels.[1]
While mice placed on a choline supplemented diet showed an increase in the proportions of the bacterial taxon Clostridiales in the gut, DMB induced a decrease in the proportions of this taxon.[1]
Mice showed no evidence of toxicity to chronic (16-week) DMB exposure.[1][2]
Occurrence
DMB is found in some balsamic vinegars, red wines, and some cold-pressed extra virgin olive oils and rapeseed oils.[1]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Wang, Zeneng; Roberts, Adam B.; Buffa, Jennifer A.; Levison, Bruce S.; Zhu, Weifei; Org, Elin; Gu, Xiaodong; Huang, Ying et al. (December 2015). "Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis". Cell 163 (7): 1585–1595. doi:10.1016/j.cell.2015.11.055. PMID 26687352.
- ↑ , Manfred Dr"Process for the production of pure neohexanol" patent EP0081050A1, issued 1983-06-15
 |

