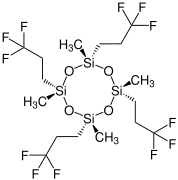
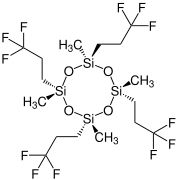
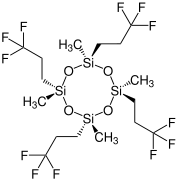
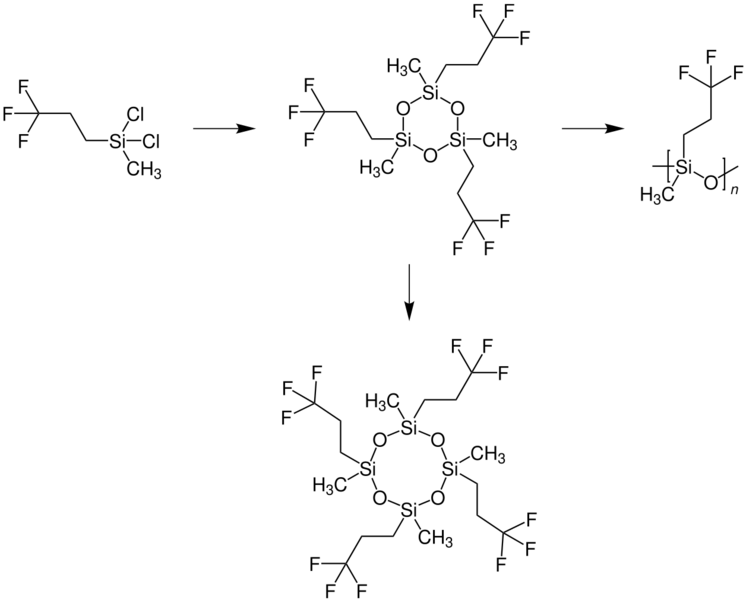
Chemistry:Tetrakis(trifluoropropyl)tetramethylcyclotetrasiloxane
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C₁₆H₂₈F₁₂O₄Si₄ | |
| Molar mass | 624.71 |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H302, H312, H315, H319, H332, H335, H410, H413 | |
| P261, P264, P264+265Script error: No such module "Preview warning".Category:GHS errors, P270, P271, P273, P280, P301+317Script error: No such module "Preview warning".Category:GHS errors, P302+352, P304+340, P305+351+338, P317Script error: No such module "Preview warning".Category:GHS errors, P319Script error: No such module "Preview warning".Category:GHS errors, P321, P330, P332+317Script error: No such module "Preview warning".Category:GHS errors, P337+317Script error: No such module "Preview warning".Category:GHS errors, P362+364Script error: No such module "Preview warning".Category:GHS errors, P391, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
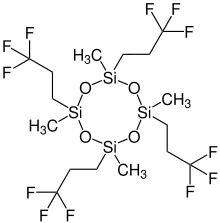
Tetrakis(trifluoropropyl)tetramethylcyclotetrasiloxane (D4F) is a chemical substance. It is a derivative of octamethylcyclotetrasiloxane (D4), but also belongs to the class of per- and polyfluoroalkyl substances (PFASs).
It occurs in four diastereomeric forms:[2]
D4F is formed as a reaction by-product in the synthesis of polymethyltrifluoropropylsiloxane (PMTFPS). The starting material is dichloromethyl(3,3,3-trifluoropropyl)silane and tris(trifluoropropyl)trimethylcyclotrisiloxane (D3F) is an intermediate.[3]
It has been detected in wastewater,[4] sewage sludge[5] as well as in biosolid-amended soils.[6]
References
- ↑ "Cyclotetrasiloxane, 2,4,6,8-tetramethyl-2,4,6,8-tetrakis(3,3,3-trifluoropropyl)-" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/67934#section=Safety-and-Hazards.
- ↑ Zhi, Liqin; Sun, Hongyu; Xu, Lin; Cai, Yaqi (2021-01-19). "Distribution and Elimination of Trifluoropropylmethylsiloxane Oligomers in Both Biosolid-Amended Soils and Earthworms" (in en). Environmental Science & Technology 55 (2): 985–993. doi:10.1021/acs.est.0c05443.
- ↑ OECD: Synthesis Report on Understanding Side-Chain Fluorinated Polymers and Their Life Cycle, Figure 5.1
- ↑ Huang, Zichun; Xiang, Xiaoling; Xu, Lin; Cai, Yaqi (2020-10-15). "Phenylmethylsiloxanes and trifluoropropylmethylsiloxanes in municipal sludges from wastewater treatment plants in China: Their distribution, degradation and risk assessment" (in en). Water Research 185: 116224. doi:10.1016/j.watres.2020.116224.
- ↑ Xiang, Xiaoling; Liu, Nannan; Xu, Lin; Cai, Yaqi (2021-11-01). "Review of recent findings on occurrence and fates of siloxanes in environmental compartments" (in en). Ecotoxicology and Environmental Safety 224: 112631. doi:10.1016/j.ecoenv.2021.112631.
- ↑ Zhi, Liqin; Sun, Hongyu; Xu, Lin; Cai, Yaqi (2021-01-19). "Distribution and Elimination of Trifluoropropylmethylsiloxane Oligomers in Both Biosolid-Amended Soils and Earthworms" (in en). Environmental Science & Technology 55 (2): 985–993. doi:10.1021/acs.est.0c05443.
 |