Chemistry:Sulfine
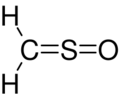
| |
| Names | |
|---|---|
| Preferred IUPAC name
Methylidene-λ4-sulfanone | |
| Other names
sulfine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| CH2OS | |
| Molar mass | 62.09 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sulfinylmethane or sulfine is an organic compound with molecular formula H2CSO. It is the simplest sulfine. Sulfines are chemical compounds with the general structure XY=SO.[1] IUPAC considers the term 'sulfine' obsolete,[2] preferring instead thiocarbonyl S-oxide; despite this, the use of the term sulfine still predominates in the chemical literature.
Substituted sulfines
The parent sulfine H2CSO is very labile, whereas substituted derivatives are more conveniently isolated.
One route is a variant of ketene synthesis, in which a sulfinyl halide reacts with a hindered base. For example, syn-propanethial-S-oxide, responsible for eye-watering effects of cutting onions, is produced so from allicin.[3]
Another route is oxidation, as with thiobenzophenone from diphenylsulfine:[4]
- (C6H5)2C=S + [O] → (C6H5)2C=S=O
See also
- Sulfene - related functional group with the formula H2C=SO2
- Ethenone
- Heterocumulene
References
- ↑ Binne Zwanenburg (1989). "Sulfine Chemistry". Phosphorus, Sulfur, and Silicon and the Related Elements 43 (1–2): 1–24. doi:10.1080/10426508908040276.
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "sulfines". doi:10.1351/goldbook.S06108
- ↑ Block E., Gillies J.Z., Gillies C.W., Bazzi A.A., Putman D., Revelle L.K., Wang D., Zhang X. (1996). "Allium Chemistry: Microwave Spectroscopic Identification, Mechanism of Formation, Synthesis, and Reactions of (E,Z)-Propanethial S-Oxide, the Lachrymatory Factor of the Onion (Allium cepa)". J. Am. Chem. Soc. 118 (32): 7492–7501. doi:10.1021/ja960722j.
- ↑ G. Rindorf; L. Carlsen (1979). "The crystal and molecular structures of the thiobenzophenone S-oxide and thiobenzophenone". Acta Crystallogr. B35 (5): 1179–1182. doi:10.1107/S0567740879005835.
 |


