Chemistry:Triarylamine
From HandWiki
In chemistry, a triarylamine has the formula NAr3 where Ar is any of several aryl groups. The parent member is triphenylamine. Triarylamines are of interest as components of molecular electronics as well as some dyes.[1]
Preparation and structure
Triarylamines can be produced by arylation of diarylamides with aryl iodides:[2]
- Ar
2NLi + Ar'I → Ar
2Ar'N + LiI
Buchwald-Hartwig coupling methods have also been applied.[3]
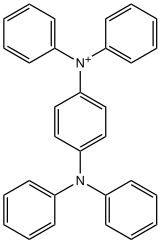
Structure of the cation ((C
6H
5)
2N)
2C
6H+
4. The phenyleneC-N distances are 1.36 pm vs 143 pm in the neutral parent.[4]
6H
5)
2N)
2C
6H+
4. The phenyleneC-N distances are 1.36 pm vs 143 pm in the neutral parent.[4]
Many hundreds of triarylamines have been prepared. In some cases, their radical cationic derivatives have been characterized by X-ray crystallography.
References
- ↑ Wang, Jiayu; Liu, Kuan; Ma, Lanchao; Zhan, Xiaowei (2016). "Triarylamine: Versatile Platform for Organic, Dye-Sensitized, and Perovskite Solar Cells". Chemical Reviews 116 (23): 14675–14725. doi:10.1021/acs.chemrev.6b00432. PMID 27960267.
- ↑ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, p. 876, ISBN 978-0-471-72091-1, https://books.google.com/books?id=JDR-nZpojeEC&printsec=frontcover
- ↑ Hartwig, John F. (1998). "Transition Metal Catalyzed Synthesis of Arylamines and Aryl Ethers from Aryl Halides and Triflates: Scope and Mechanism". Angewandte Chemie International Edition 37 (15): 2046–2067. doi:10.1002/(sici)1521-3773(19980817)37:15<2046::aid-anie2046>3.0.co;2-l. PMID 29711045.
- ↑ Szeghalmi, Adriana V.; Erdmann, Marco; Engel, Volker; Schmitt, Michael; Amthor, Stephan; Kriegisch, Volker; Nöll, Gilbert; Stahl, Rainer et al. (2004). "How Delocalized is N , N , N , N -Tetraphenylphenylenediamine Radical Cation? An Experimental and Theoretical Study on the Electronic and Molecular Structure". Journal of the American Chemical Society 126 (25): 7834–7845. doi:10.1021/ja0395386. PMID 15212531.
 |

